Synthesize Eutectics and Melting Point Comparison
Purpose of the Experiment
The purpose of this experiment was to synthesize various eutectics and compare the melting points of the eutectics to their original components. In this experiment, we made four different eutectics. A mixture of choline chloride and urea was made, as well as a mixture of choline chloride and water. A mixture of choline iodide and urea was made, as well as a mixture of choline iodide and water. These eutectics were made because they were the available chemicals of the ones ordered. Of particular interest is the switching of the anion, which has not been tested much in current research. All mixtures were made in a 1:2 mole ratio of salt to hydrogen bond donor. The melting point of each is tested because eutectics are known for lowering melting points greatly. If the melting point is greatly reduced, this proves that a eutectic was made. Testing the melting points further shows how eutectics can be used in real life, such as lowering the melting points of metals used in soldering metals in electrical work. This experiment will help overall research because it will allow us to compare future melting points obtained of various eutectics and see which one lowers the melting point the most.
List of Materials and Equipment
- Choline Bromide
- Acetylcholine Bromide
- Urea
- Deionized water
- LabQuest (with temperature probe)
- Melting Station
- Hot plate
- Metal stir rods
- Stir rod
Methodology
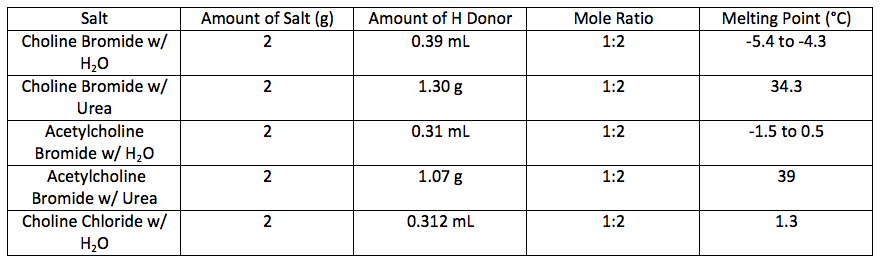
For each salt the group measured out 2 g. Stoichiometry was used to make a 1:2 salt to hydrogen bond donor molar ratio. Two samples of choline bromide were synthesized: one with 2 g of choline bromide with 0.39 mL of water, and another with 2 g but with 1.30 g of urea. Two more eutectics were made using 2 g of acetyl choline bromide for each. One sample was mixed with 0.32 mL of water and the other 2 g sample was mixed with 1.07 g of urea. The samples were then mixed by hand with a stir rod or placed on the hot plate with a metal stir rod. The samples that were mixed with water as the hydrogen bond donor turned to liquid quickly. Both samples that were mixed with urea as a hydrogen bond donor were heated to 80°C, which caused them to melt quickly, as well. All the samples were placed in a -40° C freezer for a period of two days. On the second day the melting points were collected. The temperatures of both samples using urea as a hydrogen bond donor were tested using the melting station because of their higher melting points. The temperatures of both of the water samples were tested using a LabQuest with a temperature probe because of their low melting points; both melted almost instantaneously once they were removed from the freezer. These melting point values can be found in Table 1.2

Presentation of Data
Table 1.1: Expected Melting Points of Original Components
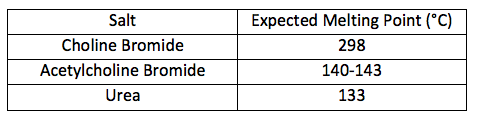
Table 1.2: Stoichiometry Results and Melting Points
Data Explanation
As shown from the tables above, the eutectics greatly decreased the melting point of the various compounds. The mixture of choline bromide with water decreased the melting point of choline bromide by nearly 300 °C. The mixture of choline bromide with urea decreased the melting point of choline bromide by about 250 °C. The mixture of acetylcholine bromide with urea decreased the melting point of acetylcholine bromide by about 100 °C. The mixture of acetylcholine bromide with water decreased the melting point of acetylcholine bromide of only 150 °C. The mixture of choline chloride with water decreased by around 260 °C from last week, and about 260 °C when compared to the melting point of choline chloride. This experimental value attained this week is believed to be a more accurate result than other weeks. The lower the melting point drops, the better the association between the hydrogen bond donor and the salt, the better the eutectic works. From this experiment, the mixture of choline chloride and water formed the best eutectic. This value will be compared in future experiments to other eutectics formed to see if it is the best eutectic tested.
Conclusion and Future Ideas
This experiment was performed to compare the melting points of various eutectics to their known component values. Throughout the experiment, it was shown that the mixture of the eutectic greatly decreased the melting points, by as much as 300°C. From here it is intended to test the melting point of other mixtures (changing the anion in the salt for future chemicals) and compare those to known values, as well as other eutectics. We have also decided to test the pH of the eutectics we have made thus far in a future experiment to test the acidity or basicity of the various eutectics. This experiment will help with the overall project because there is not a lot of research in the field of switching the anions of various eutectics and comparing their physical properties. It is intended to test the melting points of many eutectics and compare them all in future research, then moving on to other physical properties such as viscosity and pH.
