Synthesis of 5-Methyl-5-Hexen-2-one using the Organic Syntheses Method
Written by Nicole
Introduction
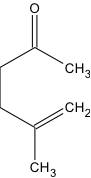
The purpose of this paper is to compare experimental methods in order to acquire the optimal procedure to synthesize the compound 5-Methyl-5-hexen-2-one. Procedures will be scrutinized according to their cost and time efficiency, as well as the complexity and thoroughness of procedure in producing product at a high yield. A detailed stepwise mechanism is indicated for 5-methyl-5-hexen-2-one. Also indicated in this paper are two methods that produce the product from experimental procedures: “Stereoselective Total Synthesis of Racemic Grandisol. An Improved Convenient Procedure” and “Reactions of Some Cyclic Ethers in Superacids.” The reasoning behind using the Organic Syntheses method over the latter two methods will be discussed. The product 5-Methyl-5-hexen-2-one acquired during experimentation occurs naturally in hazelnuts and the plant Pulchea Arabica. The compound is a component used to create a variety of fragrances including balsam, herbal, citrus, and tangerine (“5-Methyl-5-hexen-2-one”). 5-methyl-5-hexen-2-one is also used as a flavoring agent and 2.0 kg is consumed by Americans annually (Redwick and Sipes).
Preparation
The Organic Syntheses Method (1967) was chosen to be the safest, most cost-efficient way to produce 5-Methyl-5-hexen-2-one. While the percent yield produced only 51%, the cost of starting materials is relatively inexpensive compared to the other methods that are explored in this paper. The Organic Syntheses article had descriptive methods on performing the relatively easy procedure in a two-step reaction.
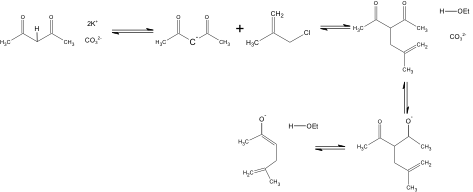
In the Organic Syntheses article, the procedure to produce 5-Methyl-5-hexen-2-one is addressed. The procedure originally produced 78.0g, but was scaled back to prepare approximately 7 grams of the compound. According to the article place 19.5g of anhydrous potassium carbonate, 11.25 g of methallyl chloride, 13.75 g of 2, 4-pentanedione and 75 ml of anhydrous ethanol in a 250 ml round-bottomed flask, which should then be refluxed for sixteen hours on a steam bath. In order for the compound to be properly refluxed, the solution needs to be boiled in the round bottom flask attached to a water condenser so that vapors are continuously condensed back into the round bottom flask for re-boiling. Then removing the condenser, replace it with a distilling head which should be topped with a condenser. 200 ml of ethanol should be distilled from the mixture. Most of the ethyl acetate that was formed as a byproduct in this reaction will also removed. 600 ml of ice water is then added to dissolve the salts and then ether is used three times to extract the mixture. The ether extracts must be washed twice with 25 ml of saturated sodium chloride solution, and then dried for 30 minutes over anhydrous magnesium sulfate and filtered. From the filtrate the solvent is then evaporated, and any residue left is distilled through a 6-in vigreux column using an oil bath kept at 190°. The Vigreux column is used when trying to separate out compounds that have similar boiling points that are close together. The closer the boiling point the longer the column. The boiling point range of the product should be 145°-155°. The procedure yields 6.5g at a percent yield of 47-52% of the product.
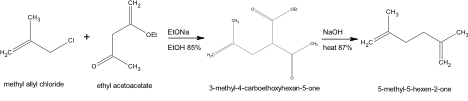
Another experimental method utilized to synthesize the product 5-Methyl-5-Hexen-2-One is described in “Stereolselective Total Synthesis of Racemic Grandisol” (1985).
The procedure denoted deviates from the method provided by a 3 fold reduction in material. A reaction must first be performed to yield the ketoester 3-methyl-4-carboethoxyhexan-5-one. The synthesis includes the mechanical set up of a 500ml three necked flask containing a mechanical stirrer, reflux condenser, a dropping funnel and a nitrogen flush. The nitrogen flush was charged with 20ml of abs ethanol. Metallic sodium (1.39g) was cut into pieces and is gradually added. The sodium was dissolved and then cooled to reach a temperature of 15°C then 8.76g ethyl acetoacetate was added. The acquired solution was stirred for 1.5 hours at room temperature then 5.49g of methallyl chloride is added gradually over 1 hour. The reaction was continually stirred overnight and refluxed for an additional 1 hour afterward. The NaCl present was extracted and disposed of through filtration. The acquired material was washed with ethanol. The ethanol solution was distilled under normal pressure. A second distillation under the reduced pressure 90-110°C/15mmHg was performed to acquire 9.5g of 3-methyl-4-carboethoxyhexan-5-in 85% yield. 5-Methyl-5-hexen-2-one may be synthesized by reacting 9.5g of the acquired ketoester 3-methyl-4-carboethoxyhexan-5-one with 76.67ml of 10% aqueous solution of NaOH. Material may be combined in a 500 ml two necked flask containing a mechanical stirrer and reflux condenser, then refluxed for 2 hours. The solution after cooling may be extracted with ether. The material containing ether and reacted product is dried by Na2SO4 and distilled to produce 5.1g of desired product, 5-Methyl-5-hexen-2-one, at 87% yield. The boiling point of product attained was 85-86°C.
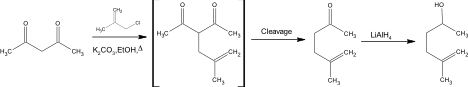
An additional procedure considered for the preparation of 5-methyl-5-hexen-2-one was “Reactions of Some Cyclic Ethers in Superacids” presented by Baig and Banthorpe.
The preparation in this proposed synthesis was similar to the preparation by the Organic Syntheses article. While 5-methyl-5-hexen-2-one is produced in this synthesis the desired product was 5-methylhex-5-en-2-ol. The synthesis started with 63.4 g (0.7 mol) of 3-chloro-2-methylpropene and 96.8g (0.7 mol) of anhydrous potassium carbonate which was added to 0.75 g (0.75 mol) of the starting material, acetyl acetone. This solution was then refluxed for sixteen hours in 500 cm3 of dry ethanol. Post refluxation the product 5-methyl-5-hexen-2-one was produced with a percent yield of 47%. Boiling point analysis then concluded that this product was relatively pure. The found boiling point of the product 5-methyl-5-hexen-2-one was 148-150oC compared to the literature boiling point of 148oC. No spectral data was run on 5-methyl-5-hexen-2-one. The procedure then continued to reduce the ketone to the alcohol 5-methylhex-5-en-2-ol by adding 7.6g (0.067 mol) of LiAlH4. Further spectral data such as mass and 1H proton nuclear magnetic resonance spectroscopy was ran on the product 5-methylhex-5-en-2-ol.
Discussion
5-methyl-5-hexen-2-one was prepared through an alkylation of acetoacetic ester by using methallyl chloride. This reaction was followed by a cleavage reaction. This resulted in a 51% yield, which was produced over the relatively short period of 17-21 hours. The experiment is relatively easy to perform, and requires no special technique or instrument. The only disadvantage to this article is that no spectral data other than the boiling point is provided. All starting materials can be obtained from Aldrich: anhydrous potassium carbonate ($0.08/g), methallyl chloride ($0.48/g), 2,4-pentanedione ($3.78/g), and anhydrous ethanol($0.08/ml). All materials are relatively inexpensive with the exception of 2, 4-pentandione, and all can be easily obtained.
Even though the Organic Syntheses Method (1967) was chosen as the best method of synthesis, due to it’s cost effectiveness and simplicity of preparation, there were several other options that were considered. The proposed experimental method provided by Rosini, Marotta, Petrini, and Ballini in Stereoselective Total Synthesis of Racemic Grandisol, was an inadequate experimental procedure in comparison to the procedure performed by Boatman due to the higher cost of experimental compounds and the complexity of procedure. The methallyl chloride necessary to procedure had a reasonable price for experimentation of 0.53/g, the ethyl ethoxide required similarly had a reasonable cost of 0.08/g. The sodium ethoxide however was not cost efficient by having a cost of 5.90/g. The procedure was also not time efficient in comparison to Boatman’s procedure. The procedure involved a complex mechanical set up, that although described in great detail, was more complicated than necessary to produce the compound 5-methyl-5-hexen-2-one. Therefore although the procedure produced a higher yield of product, 87% yield, the complexity of procedure combined with cost and time inefficiency made the method undesirable.
One of the additional procedures considered was “Reactions of Some Cyclic Ethers in Superacids” presented by Baig and Banthorpe. Baig and Banthorpe’s synthesis only briefly talks about the synthesis of 5-methyl-5-hexen-2-one, however their procedure is almost an identical synthesis to the Organic Snytheses Method. The complication of this method though is if the reaction is done to completion the product is 5-methylehex-5-en-2-ol. In this proposed method of preparation 5-methyl-5-hexen-2-one can be formed if the reaction is not done to completion, by not adding the LiAlH4 which reduces the ketone to the hexanol. All the reactants can be purchased through Aldrich: acetyl acetone ($3.71/g), 3-chloro-2-methylpropene ($2.09/ml), anhydrous potasium carbonate ($4.44/g). Baig and Banthorpe’s method was dismissed as the primary source of synthesis because the procedure was not in depth enough compared to other sources, the reaction was not cost efficient, and spectral data such as mass and 1H proton nuclear magnetic resonance spectroscopy was only included for the product 5-methylhex-5-en-2-ol rather than 5-methyl-5-hexen-2-one.
Conclusion
In order to successfully synthesize 5-methyl-5-hexen-2-one, the Organic Syntheses method was chosen due to its low cost, detailed procedure, and relatively decent percent yield at 51%. Although other methods had higher percent yields, Organic Syntheses was the most cost efficient and the most detailed. 5-Methyl-5-hexen-2-one is expected to be synthesized over the next 3 weeks producing approximately 6.5g of the product.
Citation
Boatman, S.; Hauser, C.R.; Org. Synth. 1967, 47, 87. “5-Methyl-5-Hexen-2-One”
Redwick, A.G.; Sipes, I.G.; IPCS. “Who Food Additives Series: 50: Aliphatic Secondary Alcohols, Ketones and Related Esters”
Rosini, G.; Marotta, E.; Petrini, M.; Ballini, R. Tetrahedron. 1985, 41, 4633-38. “Stereoselective total synthesis of Racemic Grandisol”
Baigh, M.A.; Banthorpe, D.V.; Carr, G.; Whittaker, D.; J. Chem Soc. Perkin Trans. II. 1989, 1981-1986. “Reaction of Some Cyclic Ethers in Superacids”
5-methyl-5-hexen-2-one 3240-09-3. (n.d.). The Good Scents Company. Retrieved March 23, 2012, from http://www.thegoodscentscompany.com/data/rw1035951.html