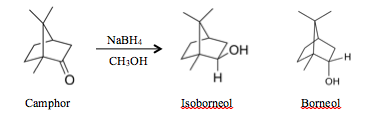
Reduction of Camphor
Written by Kevin
Abstract: This experiment will reduce camphor into isobornel with sodium borohydride. After the product is obtained, a percent yield, IR spectrum, and gas chromatography/mass spectrometry will be obtained to discuss the properties of isoborneol.
Introduction: This was a reduction reaction in whish camphor was reduced into isoborneol with the use of sodium borohydride.
Experimental Description: Camphor was allowed to be reduced into isoborneol in this reaction. Camphor was first obtained and dissolved in methanol. Sodium borohydride was slowly added in portions and the mixture was heated. After this mixture is allowed to cool down, ice water is slowly added and a precipitate is formed. This solid is then collected over a Hirsh funnel and allowed to dry. Dissolve this solid in methylene chloride then add anhydrous sodium sulfate to remove any water in solution. Heat this over a warm water bath until only the precipitate is left and all liquid is evaporated. Measure the weight of this solid that is left and obtain an IR spectrum for this compound and also run this compound through a GC/Mass Spectrometry.
Results and Discussion: Based off of the data, both isoborneol and borneol were synthesized from the reduction of camphor as seen in the gas chromatography data so the experiment was successful. The camphor had an incense-like smell and the isoborneol–borneol mixture had a similar odor. The reduction of camphor favored the production of isoborneol over borneol as opposed to an equal combination. This is because when the sodium borohydride attacks the camphor, it would attack in the endo-phase due to the steric strain caused by the two geminal methyl groups on one side of the cyclohexane ring. This can also be seen in the gas chromatography data in how the isoborneol peak is much larger than the borneol peak. Additionally from this spectroscopy, it can be seen that borneol is slightly more polar than isoborneol since isoborneol comes off the GC column with a shorter retention time. Since normal phase gas chromatography was used, the more polar compound would stick to the column longer; therefore borneol would have to be more polar to have a longer retention time. This difference in polarity is due to the location of the hydroxyl group in borneol and isoborneol. Since the hydroxyl group is in the axial axis in borneol as opposed to the equatorial axis in isoborneol, it is further away from the two geminal methyl groups so it is more exposed to attraction with the polar solvent in the gas column. The reaction is also seen as working when analyzing the IR spectra of the final product. As opposed to the camphor IR spectra, this product has an additional hydroxyl component in the IR. Therefore, this demonstrates that the product has a hydroxyl component as in isoborneol and borneol. Even though there is still a carbonyl group in the IR, the reaction did not proceed to completion but based off of the hydroxyl component, the reaction still proceeded. This carbonyl group is the main difference between the lab data and literature data but this only means that the reaction did not go to completion but the reaction still proceeded. Overall, this reduction reaction of camphor into isoborneol was completed relatively successfully.
Conclusion: The reaction overall worked well based off of both the IR and the GC/MS data. Even though the IR spectra of the final product includes a carbonyl group which is different than the literature, the reaction still proceeded in the reduction of camphor into isoborneol and borneol because of the hydroxyl peak. The gas chromatography also shows evidence of the reaction proceeding when reducing camphor into borneol and isoborneol. The peak differences show that isoborneol was preferred over borneol, due to the steric strain caused by the two geminal methyl groups on one side of the cyclohexane. Since there is still a camphor peak on the gas chromatography spectra, the reduction reaction did not go to completion. In future cases, this can be changed by including more sodium borohydride to reduce all the camphor and allow the reaction to proceed for a longer amount of time.
Experimental:
1 2 3
endo-1,7,7-Trimethyl- bicyclo[2.2.1]heptan-2-ol (2). To a stirred solution of ketone 1 (.2495g, 1.639mmol) in CH3OH (1.500mL), NaBH4 (.2520g, 6.661mmol) was slowly added. The reaction was heated and then quenched with ice water (10.00mL). The reaction was filtered and dried through a Hirsch Funnel. The solid was dissolved in CH2Cl2 (10.00mL) and this mixture was dehydrated with anhydrous Na2SO4 (.4985g, 3.510mmol). The mixture was boiled in a warm water bath to yield alcohol 2 and 3 as a white solid: IR (neat) 1733.82 cm-1, 2948.69 cm-1, and ~3300 cm-1 to ~3600 cm-1; LRMS m/z (relative intensity) 136.134 (M+, 4.16), 121.113 (62.34), 107.092 (9.48), 95.097 (25.90), 94.087 (13.45), 93.078 (100), 79.059 (12.39), 43.992 (15.66).
References: Pavia, D.; Lampman, G.; Kriz, G.; and Engel, R. Introduction to Organic Laboratory Techniques, A Small Scale Approach, Saunder’s College Publishing: Fort Worth, TX, 1998, Experiment 31, p.251
Appendix 4
Reduction of Camphor into Isoborneol and Borneol
4C10H16O + NaBH4 + 4H2O ——> 4C10H18O + NaB(OH)4
|
.2495g C10H16O |
1mol C10H16O |
4mol C10H18O |
154.28g C10H18O |
= .2528g C10H18O |
|
152.26g C10H16O |
4mol C10H16O |
1mol C10H18O |
|
.2520g NaBH4 |
1mol NaBH4 |
4mol C10H18O |
154.28g C10H18O |
= 4.111g C10H18O |
|
37.83g NaBH4 |
1mol NaBH4 |
1mol C10H18O |
Therefore, C10H16O (camphor) is the limiting reactant.
Percent Yield:
.1480/.2528 * 100 = 58.54%