Oxidation of 2-Ethyl-1,3-Hexanediol with Calcium Hypochlorite
By: Nathan Gaffney
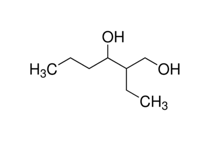
Introduction/Purpose
The purpose of this lab was to learn about the oxidation of alcohols. The starting compound was 2-Ethyl-1,3-Hexanediol, one of the hydroxyl groups was first degree while the other was second degree. The reagent used was calcium hypochlorite. Since the starting material was a diol the product outcome could have been a variety of products. That is why the results of the lab were investigated via IR to determine if the reagent calcium hypochlorite was a strong enough oxidizing agent and thus able to oxidize both hydroxyl groups, or if it was not and thus not able to oxidize either. Another possibility could have been that only one of the hydroxyl groups would be oxidized while the other remained the same. If so then that presents another question, do the degrees of the hydroxyl groups correlate with reactivity?
Procedure
The first step of the procedure was to transfer 0.5 g of 2-ethyl-1,3-hexanediol to a Erlenmeyer flask via pipette. Then a stir-bar was added and about 3 mL of glacial acetic acid. The next step was to place the mixture in an ice bath and slowly add 3 mL of calcium hypochlorite. After that the reaction was stirred at room temperature for 30 minutes while periodically verifying that the hypochlorite was present via starch-KI paper. If the paper did not turn dark purple additional drops were added to insure excess of the reagent. Once the reaction was completed it was isolated by extracting with diethyl ether and washing three time with 10 mL sodium hydroxide (1N) and once with 5 mL of brine. Finally the washed extract was dried over anhydrous sodium sulfate before being decanted into a 50 mL RB flask. The ether was then removed via rotary evaporator. An IR spectrum was then obtained and the product yield was recorded.
Results
Starting amount= 0.495 g of 2-Ethyl-1,3-Hexanediol
Percentage yield = (0.432/0.495)*100 = 87.27
Mass yield = 0.432 g
Product appearance: The product was a clear liquid.
Conclusion
The proposed product is 2-ethyl-1-hydroxy-3-heptanone. This was determined by comparing IR spectrums and basic knowledge of organic chemistry. The IR of the starting compound contained a peak at 3330.86 cm^-1 which were the two hydroxyl groups. The product’s IR gave a peak at 3376.12 cm^-1, this verified that at least one of the hydroxyl groups remained it also gave a peak at 1706.07, this indicated that a ketone was formed from one of the hydroxyl groups. As for which hydroxyl group was oxidized, that is but simple chemistry. It is based on electron density which makes the hydroxyl group reactive due to the polar bonds connected to oxygen. The secondary alcohol has more electron density because of its multiple carbon neighbors. Therefore it is more reactive than the first degree alcohol.
Before the product’s outcome was determined, many possible products were believed to have been possible. Though after analyzing the data from the experiment, the outcome defined a single product. Both hydroxyl groups could not have remained unchanged because the IR spectrum of the product showed the formation of a ketone. Both hydroxyl groups also could not have been oxidized because the product’s IR also showed an alcohol group. That is why without a doubt the final product contains both a ketone and a hydroxyl group. Since the second degree alcohol group is more reactive the oxidation could not have occurred on the first degree alcohol but rather on the second degree alcohol.
Safety
2-Ethyl-1,3-hexanediol – Eye protection needed (1)
Ca(OCl)2 – Rapid decomposition, inhalation hazard due to release of Cl2 gas (1)
AcOH – If concentrated can be corrosive to the skin and eyes (1)
2-Ethyl-1-hydroxy-3-heptanone – Eye protection needed (1)
Reference(s)
(1) http://www.sigmaaldrich.com
Additional Questions
- Since it was the secondary alcohol that was oxidized it is more reactive than the primary alcohol. The reason for this is inductive effects, the polar bonds formed by oxygen to carbon and hydrogen provide more electrons that add up to increased electron density. This creates more of a partial negative charge which allows the more electron dense hydroxyl group to be more reactive and thus more easily oxidizable.
Since calcium hypochlorite was used in excess and yet only one hydroxyl group was oxidized it is safe to say that it is not a very strong oxidizing agent.
- Using sodium hydroxide (1N) to wash during the workup helped to eliminate any leftover ions. It also helped to differentiate the aqueous layer from the product in the desired organic layer.
- No, since Na2Cr2O7 and K2Cr2O7 are both strong oxidizing agents they would have oxidized both hydroxyl groups instead of just one.
- A Lucas test could have been given to find out which hydroxyl group was oxidized. If the test had of been administered before the reaction the result would have been, the change of color from colorless to cloudy due to the second degree alcohol. If the test was administered after the reaction took place the result would have been no change in color because the compound no longer contained a secondary hydroxyl group.
