LeChatelier’s Principle Buffers
By: Selina Urbina
ABSTRACT
The effects of a strong acid and strong base were determined by measuring the pH of a buffered and unbuffered systems. Moreover, one observes the common-ion effect on a dynamic equilibrium in this experiment. One observes the way equilibrium shifts when the concentration of one species changes. This also applies to changes in temperature in the system. Overall, this experiment take a look at LeChatelier’s principle.
INTRODUCTION
The purpose of this experiment was equilibrium shifts in a system when the concentration of one species changes. The common ion effect on equilibrium is observed. The effects of temperature changes on the position of the equilibrium is also observed. (Beran). Moreover, we measured the effects of a strong acid and strong base by measuring the pH of a buffered and unbuffered system.
RESULTS
In experiment 16 part A: Metal-Ammonia Ions, one comes to find that when adding NH3 drops to .1M CuSO4 the system turns blue. Thus, [NH3] increased. The addition of ammonia caused the reaction to shift right. However, the addition of HCl (strong acid) to the system caused the reaction to shift left. [NH3] was reduced in the system.
In part B: Multiple Equilibria with Silver Ion. When .01 M AgNO3 was added to .1 M Na2CO3 a precipitate formed. The addition of 6M HNO3 caused the solution to turn clear. The addition of HNO3 caused AgNO3 to dissolve thus removing NO3 from the reaction. This reaction then shifts to the right. Moreover, the addition of HCl to the solution caused a precipitate to form.
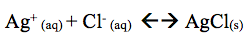
The addition of 5 drops of NH3 resulted in the precipitate sinking to the bottom. The addition of NH3 removed the silver ion. The reaction shifts left. Thus, AgCl(s) sinks to the bottom.
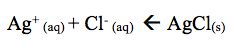
The addition of HNO3 causes gas and a clear solution to form. The results obtained reveal that when HNO3 is added a clear solution forms however, the lab manual states that when HNO3 is added to the solution it reforms the AgCl(s) precipitate. H+ reacts with NH3 to restore AgCl as shown below.
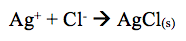
The results obtained revealed that the addition of KI to the solution showed that no reaction occurred. However, the lab manual states that when KI is added to solution the formation of silver iodide (AgI) occurs. The reaction shifts left forming AgI(s).
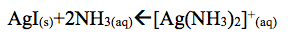
Moreover, the addition of Na2S created a light brown precipitate. In this case AgI dissolved and solid silver sulfide formed.
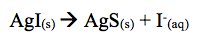
The reason AgI dissolved is because Ag2S is soluble in water. The sulfide Ion was then added to from AgS. Thus, the light brown precipitate formed when adding Na2S.
In Part C: Buffer Systems, one looks at how a strong base and strong acid effect buffered and unbuffered system.
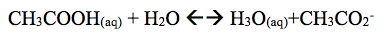
In this experiment it was found that the color universal indication of CH3COOH turned red with a pH of 4. When we added NaCH3CO2 it was close to the same red with a pH of 5. When the addition of NaCH3CO2 is added it reveals that acetic minimizes large pH scales. The pH of the solution then turns yellow with a pH of 6.5. In the buffer system it is revealed that pH of well A1 did not change. However in well A2 had a .5 change when .10M NaOH was added. A1 and A2 had little pH change because they took place in a buffered system where pH change is minimal due to the reason that a buffer system minimizes pH change. However in Well B1 and Well B2, pH change was greater. When one adds .10M HCl to the (Well B1) system a change in pH is 1.5. In Well B2 a change in pH was 3.5 when one adds .10M NaOH. This system was not a buffer system which accounts for the greater pH change in the solutions.
Part D: The color of CoCl2 turned red. When one added 5-7 drop of HCl the solution turned blue. This is called the common ion effect. Ions similar to those already present were added.
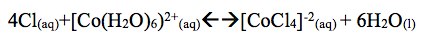
When one adds water to the solution it turned back to light red. The reaction shifts right. Turning back to red or light red. When heat was added to the solution the solution turned darker red and shifts left. The reaction absorbs heat so the equilibrium shifts left.
Materials and Methods
- .1M CuSO4
- NH3
- .1 M HCl
- 1 M HCl
- 150-mm test tube
- .01 M AgNO3
- .1 M Na2CO3
- 6 M HNO3
- .1 M KI
- .1 M Na2S
- .10 M CH3COOH
- .10 M NaCH3CO2
- .10M NaOH
Conclusion
Overall, experiment 16 gave an overview of LeChatelier’s Principle and buffers. One studied the effects of concentration and temperature changes on an equilibrium system. One noticed how a strong acid and strong base effects buffered and unbuffered systems. One also observed the common ion effect an equilibrium. In part A one observes that if NH3 is added [NH3] increases, which in turn turned the system blue. This equilibrium system shifted right. Moreover the addition of HCl to the system caused the equilibrium system to shift left. Thus, reducing the [NH3]. Moreover, when .01 M AgNO3 was added to .1 M Na2CO3 a precipitate formed. In part C we observed the changes in pH for HCl and NaOH in a buffer system compared to an unbuffered systems.
References
Beran, Jo A. “EXPERIMENT 15.” Laboratory Manual for Principles of General Chemistry. New York: Wiley, 2004. 201-208. Print.
