Gravimetric Determination Of Soda Ash – Lab Report
By: Hector Manuel Gonzalez Avalo, lab partner; Benjamin Horrocks
Introduction:
Soda Ash (Na2O sodium oxide) is derived from sodium carbonate. Sodium oxide is a salt used mostly for ceramics and crystal making.
Among other applications of soda ash is its application in “water glass”, a raw material that contains sodium oxide.
Water glass is used as a source of sodium for several industrial products, such as; laundry detergents, binders and adhesives.
As a chemist the determination of the amount of this compound allows to judge the quality of any raw material before its use in the manufacturing of finished goods.
Abstract:
During this experiment HCl and NaOH solutions were prepared. NaOH was standardized using KHP, and HCl was standardized with the known concentration of the standardized NaOH before performing the determination (titration) of the amount of Na2O.
Before titrating the soda ash with NaOH, the soda ash was set to boil with the HCl to evolve the carbon dioxide that the salt produces when the acid is added; this action diminishes titration errors enormously.
The experiment was finish in two different sessions.
Procedure:
- Prepare a saturated solution of NaOH by taking sodium hydroxide and dissolving it in a test tube. The dilution of NaOH is extremely exothermic, it is important to cool down the solution using a beaker filled with water to place the tubes when they get hot. Make sure the water level of the beaker is low to avoid water to get into the test tube in case it is not stoppered.
- After no more of NaOH can dissolve, take the test tube and used the centrifuge to separate the carbonate and the actual NaOH solution.
- Put 1000 mL of water into a beaker and set to boil. Use half and half of the boiled water to dilute more the centrifuged solution; use a Pasteur pipet to subtract the diluted NaOH without touching the bottom of the test tube, that’s where the carbonate precipitate is. First add 500 mL of water to the volumetric flask along with the NaOH and then 500 mL more to complete the process. It is important to let the water cool down.
KHP will be used to standardized the NaOH
- Dry 3-5 g of primary standard KHP.
- Weight three samples of about 0.8 g of KHP and pour inside three individual 250 mL Erlenmeyer flasks.
- Add approximately 50 to 75 mL of distilled water to each Erlenmeyer containing the KHP, and add two drops of 0.1% w/v phenolphthalein as an indicator.
- Titrate the KHP with NaOH until getting a persistent pink color.
- Calculate the normality of the NaOH using the formula present in the hand out provided by the lab instructor.
After knowing the concentration of the NaOH it’s time to prepare the HCl solution and titrate with the NaOH to know its concentration:
- Pipet 25 mL of HCl to three 250 mL Erlenmeyer flask.
- Add two drops of phenolphthalein and titrate using the NaOH of known normality. Titrate until getting a persistent pink color.
- Calculate the normality of the HCl
Since the concentration of HCl and NaOH are now known; we proceed to determine the amount of soda ash in our unknown sample:
- Dry the soda ash unknown for two hours at 140-Celsius degrees.
- Weigh three sample of the soda ash unknown of about 0.2-03 g each.
- Pipet 50 mL of the previously titrated HCl inside each Erlenmeyer containing the soda ash since some CO2 will evolve from the addiction use a hot plate to boil each Erlenmeyer with the HCl and soda ash for about 3 to 4 minutes.
- Cool down the samples and add two drops of phenolphthalein, titrate using the NaOH until getting a persistent pink color.
- Calculate the amount of Na2O obtained using the equation provided in the lab handout.
Calculations and observations:
Unknown number: 260
KHP weighed: 5.0053 g
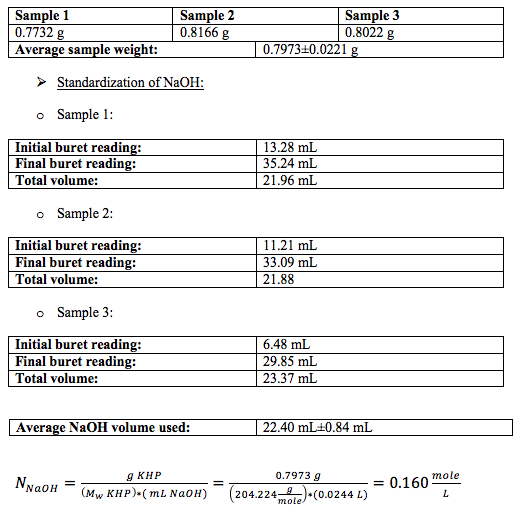
For the calculation of the normality of NaOH I used the mean volume for all the titrations, and converted it to liters. I did not have to convert the molecular weight of KHP to mg per mole because I converted the mean volume of NaOH to liters.
- Standardization of HCl with NaOH:
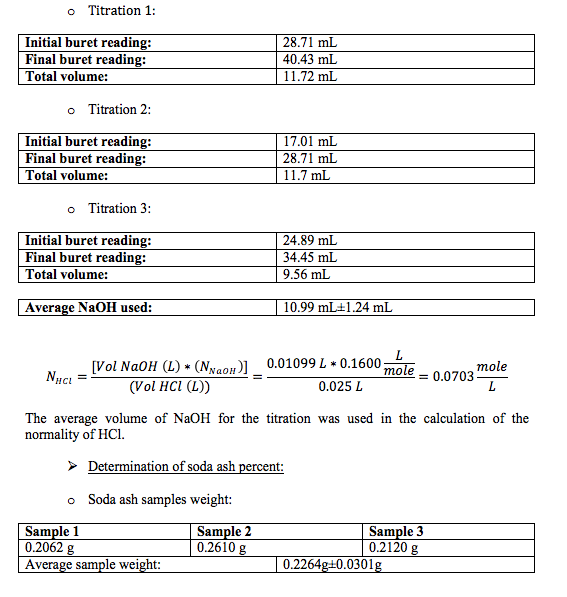
Standardized NaOH and HCl solutions were used to determine the amount of soda ash in the unknown.
During this lab we messed up a couple of titration and had to re-do them.
The information that is cross-lined in my notes is actually right, I got confused when I was explaining something to my lab partner but after some thinking and comparing his data with mine, I realized my data was actually right.
I Re-typed my lab information in another sheet so it will look more neat, however, I attached my old data.
Conclusion:
This lab was quite long because we had to dry the KHP and the soda ash, and also make in total 9 titrations without taking into account those we messed up and had to re-do to get a better result.
HCl and NaOH solutions were prepared aiming for a molarity of 0.1 M, but the molarity of NaOH went a little bit higher (0.160 M) and the molarity of HCl went a little bit low (0.0703M). I think the reason of getting lower results for the molarity of each solution is because I used the average volume of NaOH required for each set of titrations.
A higher accuracy could have been obtained if we had use more samples in each titration, but for the sake of time just three in each step was done.
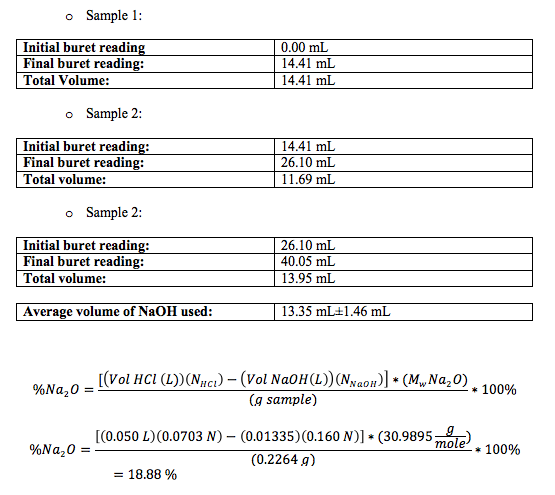
The final result for the percent amount of soda ash was of 18.88%, lower than my lab partner result. Again, I think this is because I used the mean volume of NaOH used in the titration of soda ash with HCl.
I am not one hundred percent sure if using the average value for each calculation is right or wrong, but my lab partner and I got somewhat similar results, just with small variations.
Bibliography:
United States Geological Survey – Soda Ash Statistics and Information
Source: Internet
Link: http://minerals.usgs.gov/minerals/pubs/commodity/soda_ash/
Encyclopedia Britannica – Water glass
Source: Internet
Link: http://www.britannica.com/EBchecked/topic/234888/glass#ref151656
CHEM 2260 Handout – Gravimetric Determination of Soda Ash
Source: e-learning
