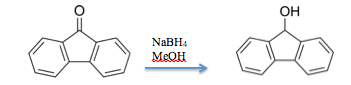
Fluorenone Reduction
Written by Kevin
Abstract: This experiment will observe the reduction of fluorenone to fluorenol (Fluorenone Reduction) through thin-layer chromatography. This reduction will be monitored at different time intervals and under different solvent concentrations to be compared with two reference solutions of fluorenone and fluorenol. In the end, a general trend will be seen between the solvent system used with different concentrations of MeOH and THF and the total length of time for the reaction to go to completion.
Experimental Section: Fluorenone is allowed to reduce into fluorenol with NaBH4. Throughout the reaction, samples of the solution are taken at different time intervals to determine whether or not the reaction has gone to completion. These samples will be placed on the baseline of TLC plates. In addition, solutions of fluorenone and fluorenol will also be placed on the baseline to serve as reference solutions to determine whether or not the reaction has gone to completion. These TLC plates will be put in a development chamber with different concentration mixtures of MeOH and THF. After plate development, allow the plate to dry and observe whether or not the reaction has gone to completion by viewing the plates under a UV lamp.
Results and Discussion: Each person was given a different concentration combination of MeOH and THF as the solvent then the reaction times are compiled to help observe a general trend based on concentrations and time. The data is as follows:
| Solution Concentrations | Time (Min. averages between all sections) |
| 100% MeOH | 2.625 |
| 80/20 MeOH/THF | 4.889 |
| 75/25 MeOH/THF | 9.500 |
| 50/50 MeOH/THF | 20.818 (My data: 15.000 minutes) |
| 25/75 MeOH/THF | 25.000 |
| 20/80 MeOH/THF | 52.429 |
| 100% THF | 69.000 |
Although there are some outliers that could have skewed the results, the general trend is that as the concentration of MeOH decreased, the reaction time increased. This is because during the reaction, a hydrogen from the borohydride first attaches onto the carbon in the carbonyl group of fluorenone which would leave the oxygen with a negative. In the presence of MeOH, the oxygen would be able to steal the hydrogen from the alcohol group on MeOH which would speed up the reaction as opposed to taking another hydrogen from another borohydride.
Conclusion: This experiment focused mainly on the technique of thin-layer chromatography (TLC). Overall, the TLC went well since a general trend can be seen between the concentrations of the solvent and the time it takes for the reaction to finish across all data. However, throughout individual data and even looking across the board, there are many outliers that did not fit this general trend. The main problem with the TLC technique is how it is difficult to obtain samples at exact times since it would take many seconds both before and after to obtain a sufficient amount of sample to spot the TLC plate. In addition, sometimes the TLC was difficult to distinguish since either the dots were too large or too small and too close to the others around it. Therefore, the technique of obtaining the right amount of sample to spot the TLC is also one that people would have to work on many times to master. The TLC technique can best be improved next time by having enough spotters prepared and getting an idea of how much sample to spot before spotting the sample.
Post-Lab Questions:
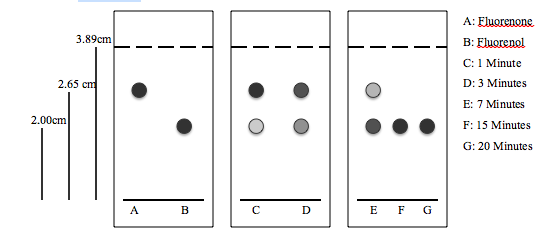
- TLC Plates
The fluorenone and fluorenol samples serve as reference spots to determine when the reaction goes to completion. As the reaction proceeds, the spot at the fluorenone location diminishes whereas the spot at the fluorenol location darkens. In the end when the reaction is complete, there is only a dark spot at the fluorenol.
2. For the solvent solution with 50:50 MeOH/THF, it took 15 minutes for the reaction to go to completion. Across all lab data, the average time for this specific solvent solution was 20.818 minutes. When looking at different concentrations of the solvent solution and comparing it to the time it takes for the reaction to goes to completion, the general trend is that as the concentration of MeOH increases, the reaction time decreases. This is because during the reduction of fluorenone to fluorenol, a hydrogen from the sodium borohydride first attacks the carbon in the carbonyl group of fluorenone, leaving the oxygen with a negative charge. The oxygen is then protonated with another borohydride compound. If MeOH is present, this protonation can occur with the oxygen stealing the hydrogen from the hydroxide group on methanol. This is more favorable since the oxygen in the hydroxyl group is highly electronegative which makes the hydrogen easy to remove.
3. Rf Values
| A: Fluorenone | 0.681 |
| B: Fluorenol | 0.514 |
4. The closer the Rf values are to one, the higher the spot travels. Since the silica gel is polar, it would hold onto the polar compounds more strongly so if a compound is polar, it would not travel as high up the TLC place during development. In this experiment, since fluorenone has a higher Rf than fluorenol, it travels further up the TLC plate which means that fluorenone is less polar than fluorenol.