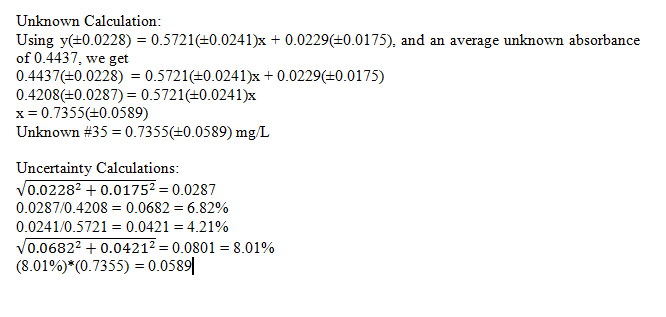Determination of Phosphates in Water
Determination of Phosphates in Water
By: David Gould
In this experiment, we determined the concentration of phosphorous in an unknown sample. This was done using standards, and then creating a calibration curve to find the unknown concentration using its absorbance. The unknown, number 35 was found to be 0.7355(±0.0589) mg/L phosphorous, which is a relatively small error.
Procedure: A Combined Reagent was prepared using 50 mL of 5N sulfuric acid, 5 mL of potassium antimonyl tartrate, 15 mL ammonium molybdate, and 30 mL ascorbic acid. Six standards were then prepared, each containing 4 mL of the combined reagent and 25 mL of sodium phosphate monobasic monohydrate. The same was done for unknowns, and a reagent blank using deionized water. Each solution was then allowed to sit for ten minutes to completely react.
Each sample was measured for absorbance in a UV-Vis spectrophotometer at a wavelength of 880 nm, with the results recorded below. The combined reagent was disposed of, and the phosphorous standards capped and stored for a later experiment.
Results:
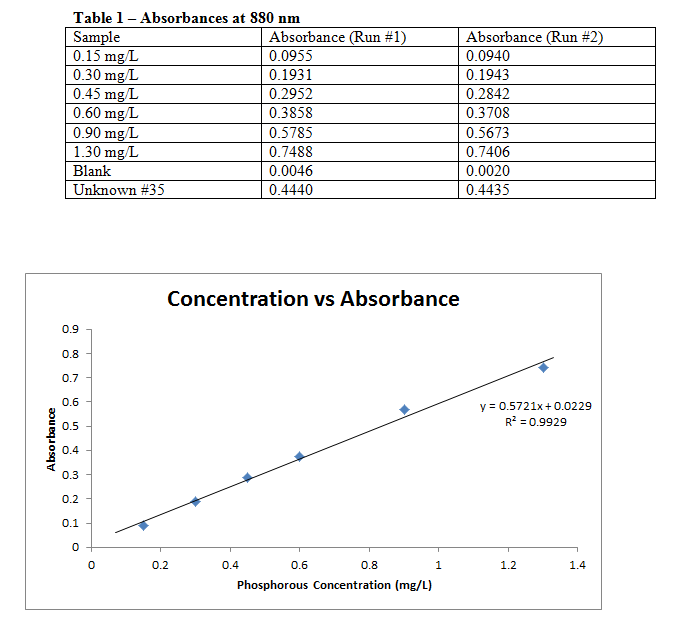
LINEST Data:
Slope: 0.572078 Intercept: 0.022927
± 0.024117 ± 0.017544
r2: 0.992941 s(y): 0.022795
F: 562.6794 Degrees of Freedom: 4
Regression SS: 0.292364 Residual SS: 0.002078
Best fit line with uncertainties: y(±0.0228) = 0.5721(±0.0241)x + 0.0229(±0.0175)
Comments: The calibration curve lines up very well with the data points, and the R2 value reflects this as well. Due to this one can say with confidence that the data is quite accurate.
It can also be seen from the unknown calculations that there is roughly an 8% error in the concentration. This is a pretty small error, again showing accuracy in the obtained results. Although this shows decent results, they could be improved further by perhaps using more runs, or more precise instruments.
References:
Cissell, K. Chem 314 Lab Manual, University Center, MI, 2014
ScienceLab.com/msds. Accessed February 15, 2014
Appendix: