Gas Chromatography to Determination of Percent Alcohol in Crest Pro Health and Listerine Mouthwashes
Determination of Percent Alcohol in Crest Pro Health and Listerine Mouthwashes via Gas Chromatography
By: Kiley Morgan, JT Ray, Amy Sizemore, Andrew Puetz
Introduction
Mouthwash is an antiseptic solution used as a hygienic product for oral health. This antiseptic can kill bacteria, prevent and combat plaque buildup, gingivitis, and bad breathe. The two mouthwashes analyzed in this experiment are Crest Pro Health Mouthwash and Listerine Mouthwashes. These two mouthwashes contain different active ingredients. Listerine’s active ingredients to kill bacteria are combination of eucalyptol, menthol, methyl salicylate, and thymol, shown in Figure 1.1 The use of alcohol as an active ingredient can burn the mouth and in some cases cause mouth sores. Crest Pro Health’s active ingredient an antiseptic, Cetylpyridinium Chloride (CPC), that can permeate mucous membranes and kill bacteria. CPC does not have the harsh effects that alcohol has on the mouth.2 Figure 2 shows the structure of CPC.
In this experiment, the percentage of alcohol in Listerine will be determined, and the absence of alcohol in Crest Pro Health will be confirmed via Gas Chromatography.
Methods
Gas chromatography (GC) can be used to chemical components in a sample. GC is a very popular separation technique, because it has a broad applications, and high measurements of sensitivity and selectivity. In GC, the components of the mixture are competitively distributed between the mobile and stationary phase. The mobile phase consists of a gas stream, referred to as the carrier gas. The stationary phase, which is packed into the column, is a solid, typically silica gel, in adsorption chromatography, and it is a liquid in partitioning chromatography.
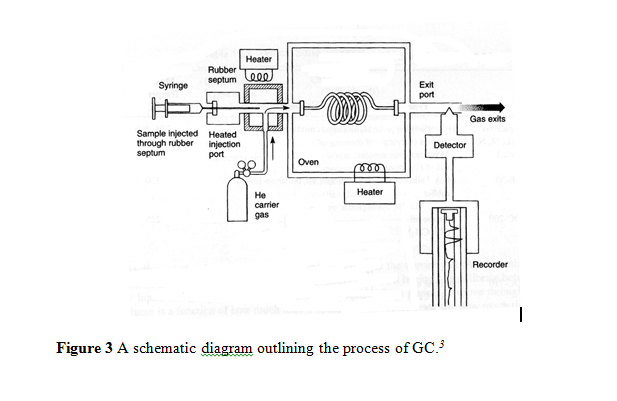
A diagrammatic scheme, Figure 3, is shown bellowing outlining the process of GC, which begins by introducing the sample to the carrier gas at the beginning of the column. This is typically at a high temperature, so the sample will be quickly evaporated. Ideally, the time it takes for the components of a sample to run through the column is inversely proportional to the column temperature. As the components of the sample leave the column in the gas phase, a detector produces a signal proportionate to the concentration of the component, resulting in a chromatogram. If the components are chemically different enough from each other, a separation has occurred.
Theoretically, GC can be used to determine the components of a sample, because under fixed temperatures and carrier gas flow rates, the retention time of a substance should be the same. It should be noted that the retention time of a substance may not be unique to just that substance, which is why GC is often paired with Mass Spectroscopy to give concrete results.4
Procedure
Materials
Glassware: Reagents:
100mL volumetric flask Listerine Mouthwash
Micropipette (various sizes) Crest Pro-Health Mouthwash
Auto-sampler vials 99.9% Ethanol
Beakers (various sizes) Distilled Water
Graduated Cylinder
Experimental Procedure
The standard alcohol solutions were prepared with 99.9% ethanol. The amounts of ethanol used according to % volume of alcohol are shown below in Table 1. Each amount was measured in a graduated cylinder and added individually to a 100 mL volumetric flask and filled with distilled water to mark. An aliquot if 750 µL of each standard solution was added to a 1 mL vial with a micropipette.
|
% Volume Alcohol |
99.9% Alcohol (mL/100mL) |
|
4.97 % |
5 mL |
|
9.95 % |
10 mL |
|
19.9 % |
20 mL |
|
33.2 % |
33 mL |
Table 1 The amount of 99.9% ethanol added to volumetric flasks to obtain % volume alcohol
A 1:10 dilution of each mouthwash was prepared and added to a 1 mL auto-sampler vials. The following parameters were programed into the CG:
Initial Temperature: 40 ºC
Final Temperature: 140 ºC
Ramp 20º/min
Hold Time: 4 minutes
Detector Limit: 10V
The sample vials were placed into the auto-sampler and the GC was programmed to analyze the samples. After the samples were run, the retention times and peaks were recorded and identified. The % alcohol was determined for Listerine.
