Determination of Manganese in Steel
By: Juno Kim and Nicole
Introduction
The goal of this experiment was to determine the mass percent of manganese in an unknown steel sample using methods of visible spectroscopy and volumetric analysis. The two methods were then analyzed and compared to decide the better method for determining the composition of Mn in the unknown steel. In both methods, the unknown steel was digested in hot concentrated Nitric acid, HNO3, and analyzed for transition metals. An accurate analysis of steel composition is important because the mass percent of carbon and transition metals in the steel determine its properties such as strength, conductivity, ability to be altered by heat, and corrosiveness that ultimately decide the steel’s usage. An alloy is a mixture of two or more elements, one of them being a metal, and steel is an alloy of iron containing small amounts of transition metals. Adding carbon to iron creates steel which has versatile uses for its general properties.
Pertaining to this lab, knowing the composition of steel reveals the best form of usage. For example structural steels contain alloying elements like Mn that can be used to produce complex structures and machine parts while tool steels have higher carbon mass percentage and contain alloying elements such as chromium. Compared to iron, steel is tougher with high strength and has the ability to greatly alter form through heat treatment. Adding Chromium to steel produces stainless steel that resists corrosion and adding silicon to steel creates silicon steel used for electronic purposes. The composition of steel must be determined and double checked prior to its intended use to avoid consequences as large as a bridge collapsing due to the use of inadequate steel. The methods of determining the composition of steel can also be used to analyze the strength and durability of already standing structures that have been subject to corrosion and weathering as well. The main objective of the experiment was to determine the manganese composition of the steel unknown by the methods of standard additions, involving visible spectroscopy, and volumetric analysis, involving back titration.
Experimental Methods
I. Standard Addition
The standard addition method used visible spectroscopy to determine the concentration of
manganese in the unknown steel sample. Standard addition is used to account for the potentially interfering ions from other transition metals.2 To start with, the sample of unknown steel was digested in hot nitric acid. Precisely 1.0437g of steel unknown and 50 mL of 4M nitric acid, HNO3, were added to a 250mL beaker and brought to a gentle boil. The beaker was covered with the watch glass to avoid losing its contents through splattering. It took close to an hour for all of the unknown steel to dissolve so excess 4M HNO3 was added during the digestion to displace the evaporated liquid and keep the volume close to 50 mL. After digestion, 1.0 g of ammonium peroxydisulfate, [(NH4)2S2O8], was slowly added to the beaker and put to boil for 15 minutes. During the boil, peroxydisulfate oxidizes any carbon in the sample in the reaction shown below:
2S2O82- + C + 2H2O -> CO2 + 4SO42- + 4H+
Following the procedure, 0.1 g of sodium bisulfate (NaHSO3) was added while heating and the resulting solution was left to cool to room temperature and transferred to a 250 mL volumetric flask where it was diluted with distilled water to the mark. Note that NaHSO3 solution was added to reduce any permanganate that may have formed through this reaction:
5HSO3– + 2MnO4– + H+ -> 2Mn2+ + 5SO42- + 3H2O
Following the steel digestion, the standard Mn solution was prepared. For that 100 mg of Mn was dissolved in 10 mL of 4M HNO3 and put to boil to remove nitrogen oxides. The resulting solution was diluted to the mark with DI water in a 1 L volumetric flask.
After the necessary solutions were prepared, standard additions took place. Total of seven samples were prepared for the spectroscopy and in each sample 20 mL aliquot of the digested steel was put into a 250 mL beaker. Then 5 mL of 85% phosphoric acid was added to eliminate iron(III) as a source of interference when taking the spectroscopy. Samples of standard Mn2+ and solid potassium periodate were added to the beaker according the table I provided below:
Table I: Calibration Standard Sample Volumes
|
Sample |
Steel |
H3PO4 |
Standard Mn |
KIO4 |
|
1-blank |
20 mL |
5 mL |
0 mL |
0g |
|
2 |
20 mL |
5 mL |
0 mL |
0.4g |
|
3 |
20 mL |
5 mL |
1 mL |
0.4g |
|
4 |
20 mL |
5 mL |
2 mL |
0.4g |
|
5 |
20 mL |
5 mL |
3 mL |
0.4g |
|
6 |
20 mL |
5 mL |
4 mL |
0.4g |
|
7 |
20 mL |
5 mL |
5 mL |
0.4g |
Upon heating, KIO4 oxidizes Mn2+ to a permanganate ion in the reaction given below:
2Mn2+ + 5IO4– + 3H2O -> 2MnO4– + 5IO3– + 6H+
Each of the samples were boiled for 5 minutes and cooled before being diluted in a 50 mL volumetric flask. Then, using the UV-Visible spectrometer, absorbance at the max wavelength for permanganate ion was measured. The max wavelength for the permanganate ion is 525 nm and the analyzers are designed to measure the absorbance in a particular wavelength band1. Small aliquots of each sample were added to a cuvette to measure the absorbance and a linear graph was expected with no absorbance value greater than 1.0 for any of the samples. At the end, the absorbance of the blank solution, containing no Mn or KIO4, was deducted from the other samples’ absorbance values. The line of best fit for the plot of concentration of added Mn2+ vs. the absorbance was drawn to find the x-intercept which represented the concentration of Mn in the unknown steel sample. The concentration of added Mn2+ was calculated by using the concentration of the standard Mn solution as shown below:
100.0 ppm * (mL of Mn added/50 mL) = concentration of Mn in ppm
II. Volumetric Analysis
Determination of Mn in the unknown steel through volumetric analysis involved titrations. A standard potassium permanganate (KMnO4), standard Ferrous Ammonium Sulfate (Fe(NH4)2(SO4)2), and an unknown steel sample were prepared in lab for the titration of the unknown steel sample.
The KMnO4 solution was prepared by glass filtering 100 mL of 0.1 M KMnO4 solution through a sintered glass filter. The resulting solution was then transferred to a 1 L volumetric flask and diluted to the mark with DI water. To standardize this solution, solid sodium oxalate was put to dry in an oven for an hour. Then three 100 mg samples of dried sodium oxalate were transferred to 250 mL beakers along with 100 mL of 0.9 M sulfuric acid (H2SO4) and heated. While heating, a burette was filled with the permanganate solution and its initial volume was recorded. A single drop of the permanganate solution was added to each of the beakers while heating and the titrations commenced once the pink color from the permanganate disappeared. The reappearance of the pink color marked the end of titrations. The reaction of permanganate with oxalate is as follows:
2MnO4– + 5C2O42- + 16H+ -> 2Mn2+ + 10CO2 + 8H2O
Using stoichiometry, the concentration of MnO4– in the solution was calculated using the formula:
0.100g C2O4– * (1 mol/88.01928g C2O4–) * (2mol MnO4–/5 mol C2O4–) *(1/L of MnO4– used)
Taking the average of the three trials yielded a MnO4– concentration of 0.0148 M in the standard solution.
For the preparation of standard Fe(NH4)2(SO4)2 solution, about 12 grams of ferrous ammonium sulfate hexahydrate were added to a 1L volumetric flask and dissolved in 1:20 sulfuric acid (H2SO4). The solution was diluted to the mark with 1:20 H2SO4. For the standardization process, 25 mL of 1:30 nitric acid (HNO3) was added to a 250 mL Erlenmeyer flask using a volumetric pipette. Then 25 mL of Fe(NH4)2(SO4)2 was added and the resulting solution was titrated with KMnO4 until the pale pink endpoint. The ferrous ions react with MnO4– in the redox reaction given below:
MnO4– + 5Fe2+ + 8H+ -> Mn2+ + 5Fe3+ + 4H2O
Using stoichiometry, the concentration of the ferrous ion, Fe2+, was calculated using the formula:
(Volume of KMnO4 used) * 0.0148M KMnO4 * (5 M Fe2+/1 M MnO4) * (1/volume of sample)
Taking the average concentrations of four trials yielded a Fe2+ concentration of 0.0466 M in the standard solution.
For the preparation of steel unknown sample, 0.2351 g of the unknown steel sample was added to a 250 mL beaker. The manual states a whole gram of the unknown steel should be used, but after a few failed trials with the first method, only 0.2351 g of the unknown steel was left for analysis. Then 50 mL of nitrous acid free 1:3 HNO3 was added to the beaker and the contents were put to a gentle boil under a watch glass cover. Once all the steel dissolved, the beaker was removed from heat and 0.5 g of sodium bismuthate (NaBiO3) was added. After the addition of NaBiO3, the contents were boiled for another five minutes which after, the solution turned purple so there was no need to add additional grams of NaBiO3. The resulting purple solution was removed from heat and drops of sodium sulfite (NaSO3) were added until the purple color disappeared (3 drops used). Then the solution was put to boil and became rust orange in color after 5 minutes. The beaker was cooled in an ice bath and allowed to chill and after, 0.7 g of NaBiO3 was added to form a solid NaBiO3 inside a purple solution. The reaction of bismuthate with manganese ion is shown below:
2Mn2+ + 5BiO3– + 14H+ -> 2MnO4– + 5Bi3+ + 7H2O
To transfer the solution, a sintered glass filter was used instead of filter paper which the Mn could react with. The filter was washed with 1:30 HNO3 and the solution inside the beaker was filtered into a flask. After filtration 4 mL of 85% phosphoric acid (H3PO4) was added to the filtrate and mixed. The resulting solution was then transferred to a 100 mL volumetric flask and diluted to the mark with 1:30 HNO3.
After the necessary solutions were prepared, the titration of the unknown steel 64 commenced. 25 mL of the (Fe(NH4)2(SO4)2) solution as well as 25 mL of the steel unknown solution were added to an Erlenmeyer flask using a volumetric pipette. The purple color disappeared as the steel unknown reacted with the ferrous ions. Then the solution was back titrated to the pink endpoint with the standard KMnO4 solution. In this back titration, an excess of standard (Fe(NH4)2(SO4)2) was added to the steel unknown, turning the color clear as the ferrous ions reacted. Then the excess (Fe(NH4)2(SO4)2) was titrated with the standard KMnO4 until the endpoint which was signaled by the reemergence of the pale pink color. Once all the Fe2+ ions have reacted, MnO4– remained in the solution to signal the end of the back titration with the pale pink endpoint. The net ionic equation is:
MnO4– + 5Fe2+ + 8H+ -> Mn2+ + 5Fe3+ + 4H2O
The percentage Mnin the steel unknown was calculated by the formula below:
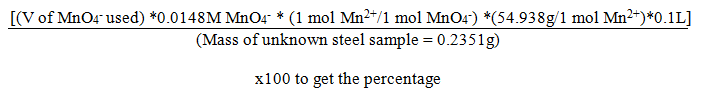
Results
The results from the two different methods were synchronized. The Mn contents of all the unknowns vary from 0.10% to 1.00% so both methods gave an acceptable result.
The Beer’s law states that absorbance is proportional to concentration. The calibration plot of UV-Vis supports the law. Beer’s law:
A = εlc
‘A’ represents absorbance with no units. ε is the molar absorption coefficient with units Lmol-1cm-1. ‘l’ represents the path length of the cuvette and ‘c’ is the concentration of the compound in solution expressed in molL-1. Another form of Beer-Lambert’s law is:
I = I010^-εcL 3
‘I’ is the transmitted intensity, varying with the length L and I0the incident intensity.2 UV and visible spectra are plots of absorbance against the wavelength in nanometers. Its absorbance is related to concentration by the Beer-Lambert’s law.4
A = log(I0/I) = εlc 4
Discussion
The weight percentage of Mn in the unknown steel sample was calculated as .51% with 95% confidence interval of 0.71 from the visual spectroscopy method. The absorption of the blank sample containing no standard Mn or KIO4 was measured to be 0.0395. The measurement of the second sample yielded absorption value of 0.4020. After subtracting the blank absorption value the first sample’s absorption was 0.3625. This was a lot higher than 0.1 absorption value the second sample should be at so the absorption values along with the steel sample volume were adjusted through division by 4. The 95% confidence interval value of 0.71 is very high. The cause of error in this method most likely resulted from the fact that the steel unknown solution had small precipitates that would not dissolve. Such error may be alleviated by letting the solution sit for a period of time until the precipitates fall to the bottom of the flask. Then samples could be taken from the top of the solution without any precipitate using a pipette. The steel unknown sample took 45 minutes longer than anticipated to digest during the lab and even after the digestion there were some particles left in the solution. This could be from impurities of the unknown steel sample. The digestion process could be sped up next time by preparing a hot HNO3 to digest the steel unknown.
The volumetric analysis also gave a Mn concentration of 0.51% in the unknown steel with the 95% confidence interval of 0.16. For this experiment, judging from the lower value for the confidence interval, this method of back titration gave more reliable concentration of Mn in the steel #64. Note that instead of using 1 g of the unknown steel sample, only 0.2351 g was used because that is what was left available. The volumes of KMnO4 used to back titrate the mixture of 25 mL unknown steel solution and 25 mL (Fe(NH4)2(SO4)2) were very consistent and consequently, they yielded consistent Mn weight percentage values in the three trials. The weight percentages of Mn for the three trials were found to be 0.52%, 0.51%, and 0.50% with the low variance of 0.02. The standard deviation, 0.1414, was calculated by square rooting the variance. The standard error, which was multiplied by 1.96 to give 95% confidence margin of error, was calculated by dividing the standard deviation by the square root of the number of trials. The confidence interval, calculated as 0.16, is still high considering the precise Mn percentage values. This could be amended by increasing the number of trials which would bring down the values (assuming the same, precise Mn percentages are calculated).
Comparing the two, both methods gave a very precise concentration of Mn in the unknown steel. The volumetric analysis had a much lower value for its confidence interval so for this experiment the back titration proved to be the better method. The visual spectroscopy method was easier in a sense that the UV-Visible spectrometer measured the absorbance for each of the prepared cuvette samples left to be graphed and analyzed. However the slight problem with this method is the fact that it relies on the line of best fit to find the concentration of Mn in the sample, making it more prone to errors by inaccurate measurements.
The volumetric analysis involved preparing two standard solutions and an unknown steel solution before the titration of the steel unknown. This method gave the experiment more control because the standard solutions were made in lab and their concentrations were calculated using stoichiometry from the redox equations. It was also easy to tell if a certain titration has gone wrong by looking at the volumetric data and noticing an outlier among the volumes used for the same titration. In this method it was critical to keep the prepared solutions from being affected by impurities. That was achieved by transferring small amounts of the standard solutions into beakers and drawing samples from the beakers. That prevented the standard solutions from being contaminated through multiple aliquots drawn by a pipette.
Conclusion:
The purpose of the experiment was to find the concentration of Mn in an unknown steel sample through the method of standard additions, involving visible spectroscopy, and volumetric analysis, where the concentration was calculated through back titration. The volumetric analysis proved to be a better method since the standard addition method’s calibration plot of the UV-Visible spectrometer yielded a faulty 0.71 margin of error under a 95% confidence level. Such margin of error is unacceptable considering the Mn concentration falls outside probable range between 0.1% and 1% after just one standard deviation away from the mean. After back titrations, the Mn concentration in the unknown steel was found to be 0.51% with 0.16 margin of error under a 95% confidence. Adding Mn to steel increases the steel’s toughness and strength and analysis of the concentration of Mn or other transition metals in steel as well as carbon can reveal the properties of the steel and its practical usage.
Interested in how manganese and other elements shaped Pittsburgh’s rise as a steel giant? Read the full story of Pittsburgh’s steel boom—and its dramatic collapse—on Steel City History.
References
1 Laverman, L.E. Experiments in Analytical, Physical and Inorganic Chemistry, 3rd Edition; p.
14.
2 Green, Don W., Perry, Robert H. Perry’s Chemical Engineer’s Handbook, 8th Edition;
McGraw-Hill: New York, 2008, p. 8-62.
3 Atkins, Peter., Paula, Julio de. Physical Chemistry, 9th Edition; W.H. Freeman and Company:
New York, 2010, p. 490.
4 Mohrig, J.M., Hammond, C.N., Schatz, P.F. Techniques in Organic Chemistry, 3rd Edition; W.
H. Freeman and Company: New York, 2010, p. 429.
