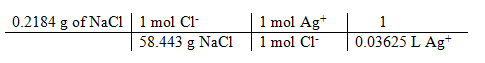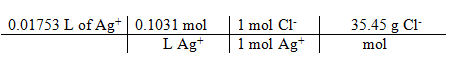Determination of Chloride Content in an Unknown Salt
Determination of Chloride in an Unknown Salt
By Diane Krehbiel
Abstract:
In this experiment, the chloride content of an unknown salt will be determined using two different methods. The first method used will be the Fajan titrimetric method which uses an adsorption indicator. The second method will use gravimetric analysis to determine chloride content. The results from both methods were very similar have average mass percent’s of 28.34% and 26.79%. This seems to indicate that the content in chloride found was correct despite the fact that the actual content is not known.
Introduction:
Two types of methods are used in this experiment to discover the mass percent of chloride in an unknown salt. The first method is called the Fajan titrimetric method. In this method, an adsorption indicator is used such as dichlorofluorescein. This is a weakly acidic dye in an ionized form. The Fajan method of titration can also be called an argentometric titration. AgNO3 is used as the titrant. The precipitate that forms will be a silver salt (“Titrimetric Analysis”). The precipitate formed will adsorb its own ions to form an adsorption layer.
In the second part of the experiment, the silver salt solid from the titration will be filtered using gravimetric filtering. After filtering off the solution, all that will be left is the precipitate on the filter paper. The filter paper will dry over the course of the week. It will also be put in the oven to ensure that any excess water be driven out of the silver salt. If there was still water present, the results would be erroneous.
This experiment is also used to compare the two methods used to discover chloride content in a salt. It is a good way to see which method is more accurate and which method is more practical. It is also good practice in taking precise measurements in order to have correct results. At the end of the experiment, the precision of the data will easily be determined, but accuracy will be much more difficult to predict because of the uncertainty of the initial chloride content.
Experimental:
In the first part of this experiment, Fajan titration will be used to discover the content of chloride in an unknown salt. To begin, prepare and standardize 0.1 M silver nitrate. To prepare this, weigh out 8.6 g of silver nitrate and dissolve it in distilled water in a beaker. Transfer the solution to a 500 mL volumetric flask and dilute to the mark with distilled water. Mix thoroughly and transfer to a brown bottle to ensure the solution does not react with the light. In my case, the professor had already prepared silver nitrate to use.
Next, standardize the silver nitrate by accurately weighing three samples of dry sodium chloride in 250-mL Erlenmeyer flasks. The weight should be between 0.2 and 0.25 g and record in notebook. If the sample is not dry, put in oven for at least 2 hours at 120°. Our samples were already dry so this step was not necessary. Dissolve each sample in 50 mL of distilled water and then add 5 drops of dichlorofluorescein to each sample. Also add 0.05 g of dextrin to each sample. In our experiment, we used dextrose instead. Titrate each of these solutions with the silver nitrate that was prepared. The initial color will be a fluorescent yellow. As the titration goes on, the solution will turn a milky white. The silver chloride flocculates right before the equivalence point. Continue to titrate drop by drop while swirling until the precipitate turns pink. Repeat this three times and average the results to find the concentration of silver nitrate.
After standardizing the silver nitrate, determine the chloride content in an unknown salt. I obtained unknown #5. Weigh three samples of the unknown salt accurately at about 0.25 g. Put each sample in a 250-mL Erlenmeyer flask and add 50 mL of distilled water. To each sample add 5 drops of dichlorofluorescein and about 0.05 g of dextrose. Titrate each sample the same as the NaCl samples were titrated. After the equivalence point is reached, record the volume and then add another mL of AgNO3 to each sample to ensure that all of the silver chloride has formed.
Next, obtain three pieces of filter paper and mark with initials and 1, 2, and 3. Weigh each paper and record weight to the nearest 0.1 mg. Filter each sample that was just titrated using gravity filtration. Rinse the Erlenmeyer flasks with minimal amount of distilled water to get all the precipitate onto the filter paper. After filtering is finished, leave samples on filter paper unopened and let dry for a week.
After a week, weigh each sample accurately and record weight in notebook. Keep the filter paper folded. Place the samples in the oven at 120°C for at least 30 minutes. I left my samples in the oven for several hours. This is to ensure that water is completely removed from the silver chloride. Water is removed at temperatures greater than 110°C but make sure that the filter paper is not charred in the oven. After the samples have been taken out of the oven and cooled for 10 minutes, weigh each sample and record in notebook. Be sure to use the same balance when weighing each time. Subtract the mass of the filter paper to obtain the mass of the silver chloride in each sample. The percent of chloride content in the unknown can then be calculated.
Data:
Unknown sample: #5
Part 1
Weight of NaCl samples:
1) 0.2184 g
2) 0.2435
3) 0.2207
Weight of dextrose:
1) 0.053 g
2) 0.058
3) 0.054
Initial Volume of Titrations:
1) 0.42 mL
2) 0.53
3) 0.39
Final Volume of Titrations:
1) 36.67 mL
2) 40.98
3) 36.97
Difference of Volumes for Titrations:
1) 36.25 mL
2) 40.45
3) 36.58
Molarity of AgNO3 calculated:
1) 0.1031 M
2) 0.1030
3) 0.1032
Average Molarity of AgNO3
0.1031
Part 2
Weight of Unknown samples:
1) 0.2312 g
2) 0.2032
3) 0.2335
Weight of dextrose:
1) 0.058 g
2) 0.051
3) 0.052
Initial Volume of Titrations:
1) 0.36 mL
2) 17.89
3) 9.51
Final Volume of Titrations:
1) 17.89 mL
2) 33.67
3) 28.00
Difference of Volumes for Titrations:
1) 17.53 mL
2) 15.78
3) 18.49
Grams of chloride calculated:
1) 0.06407 g
2) 0.05767
3) 0.06758
Mass percent of chloride calculated:
1) 27.71%
2) 28.38%
3) 28.94%
Average Mass Percent:
28.34%
Standard Deviation:
28.34% ± 0.6158
Part 3
Weight of filter paper:
1) 0.9157 g
2) 0.8890
3) 0.9207
Total weight of paper + solid (before drying):
1) 1.1976 g
2) 1.1411
3) 1.2133
Total weight of paper + solid (after drying):
1) 1.1636 g
2) 1.1053
3) 1.1805
Weight of AgCl:
1) 0.2479
2) 0.2163
3) 0.2598
Grams of chloride calculated:
1) 0.06132 g
2) 0.05350
3) 0.06426
Mass percent of chloride calculated:
1) 26.52%
2) 26.33%
3) 27.52%
Average Mass Percent:
26.79%
Standard Deviation:
26.79% ± 0.6393
Calculations:
Part 1
Final Volume – Initial Volume = Total Volume of Titration
36.67mL – 0.42 mL = 36.25 mL
Molarity of AgNO3:
grams of NaCl / formula wt = moles of Cl– = moles of Ag+
moles Ag+ / liters Ag+ titrated = [Ag+] in AgNO3 titrant
= 0.1031 M
*Repeat for other trials
Average Molarity of AgNO3:
(0.1031 + 0.1030 + 0.1032) / 3 = 0.1031 M
Part 2
Final Volume – Initial Volume = Total Volume of Titration
17.89 mL – 0.36 mL = 17.53 mL
Grams of Chloride:
[Ag+] * volume titrated = moles Ag+ = moles Cl– * 35.45 g/mole = g Cl–
= 0.06407 g Cl–
Mass Percent of Chloride:
(g Cl– / g unknown) x 100% = mass % of chloride
(0.06407 / 0.2312) x 100% = 27.71%
*Repeat for all trials
Average mass percent:
(27.71 + 28.38 +28.94) / 3 = 28.34%
Standard Deviation:
S = √ (Σ (x̄ – xi)2) / n – 1
√((27.71-28.34)2 + (28.38-28.34)2 + (28.94-28.34)2) / 3-1
= 0.6158
Part 3:
Total weight (after drying) – weight of filter paper = mass of solid AgCl
1.1636 g – 0.9157 g = 0.2479 g
Grams of Chloride:
g AgCl x 35.45 g Cl- = g Cl–
143.32 g AgCl
0.2479 g AgCl x 35.45 g Cl- = 0.06132 g Cl–
143.32 g AgCl
Mass Percent of Chloride:
(g Cl– / g Unknown ) * 100% = mass % of chloride
(0.06132 / 0.2312) * 100% = 26.52%
*Repeat for all trials
Average Mass Percent:
(26.52% + 26.33% + 27.52%) / 3 = 26.79%
Standard Deviation:
S = √ (Σ (x̄ – xi)2) / n – 1
√((26.52-26.79)2 + (26.33-26.79)2 + (27.52-26.79)2) / 3-1
= 0.6393
Results:
|
Grams of Chloride |
||
| Fajan Titrimetric method | Gravimetric Analysis method | |
| Trial 1 |
0.06407 g |
0.06132 g |
| Trial 2 |
0.05767 g |
0.05350 g |
| Trial 3 |
0.06758 g |
0.06426 g |
|
Mass Percent of Chloride |
||
| Fajan Titrimetric method | Gravimetric Analysis method | |
| Trial 1 |
27.71% |
26.52% |
| Trial 2 |
28.38% |
26.33% |
| Trial 3 |
28.94% |
27.52% |
|
Average |
28.34% |
26.79% |
Standard Deviation:
Titrimetric: 28.34% ± 0.6158
Gravimetric: 26.79% ± 0.6393
T-test = 2.333
95% confidence. There is not a significant difference between methods.
Discussion:
The results found in each part of the experiment seemed to match well. Both the titration method and the gravimetric analysis method showed very similar results of chloride content in the unknown salt #5. The average mass percent from the titrimetric method was 28.34% and the average mass percent from the gravimetric method was 26.79%. It is difficult to know which one is the closest to the actual mass percent of chloride in the salt because we had an unknown substance. If the salt was known, percent of chloride could be calculated and the percent error found based on the results of each method. The more accurate method could then be assessed.
Without knowing which method is more accurate, I would choose to just do the titrations to find out the mass percent in an unknown salt. There was not a significant difference in the answers. The titrations go faster than the gravimetric analysis. This is because it is not necessary to wait for the solutions to filter or for the precipitate to dry for a few hours in the oven. The Fajan titrations give seemingly accurate results and can be done much quicker than the gravimetric analysis. There is about the same chance for human error in both of the experiments so this does not necessarily play a role in making on method more accurate than the other.
It is unclear what my unknown salt might have been based on my results. The chloride content in my salt seemed much lower than other common possibilities. For instance sodium chloride has a mass percent of around 60% chloride. Calcium chloride also has a chloride content of around 63%. Potassium chloride has around 47% chloride in it. Magnesium chloride has an even higher mass percent of chloride in it at 74%. All these salts seem to have a significantly higher percent than my unknown salt. It is hard to know which salt my unknown could have been.
Because it appears that most salts have higher chloride contents, the titration method may have given slightly better results because it had a higher mass percent of chloride. It may have yielded a higher percent because of the room for error in the gravimetric analysis. The slight difference in numbers was most likely because all of the precipitate was not washed out of the flasks into the filter paper. There easily could have been some left and this accounts for the small difference in results between the two methods.
Conclusion:
In this experiment, the two methods used to find the chloride content of an unknown salt both yielded similar results. This indicates that most likely both methods were accurate in telling us the content of chloride. The titrimetric method resulted in a mass percent of 28.34% chloride in the salt. The gravimetric analysis came to 26.79% chloride in the salt. The difference between these two results is not significant. Both methods worked well to determine the chloride content in the salt.
Works Cited
“Titrimetric Analysis of Chloride.” Richmond University. N.p., n.d. Web. 26 Sept. 2013.