BROMINATION OF TRANS – STILBENE
AIM:
The aim of this experiment is to carry out an addition reaction using trans-stilbene and bromine reagent to produce 1,2-dibromo-1,2-diphenylethane. As most chemicals used in this experiment are toxic / harmful, PPE must be used throughout and the experiment should be carried out inside a fume hood.
THEORY:
Bromine and chlorine readily undergo addition reactions with alkenes. This practical involves the bromination of trans-stilbene using a 10% bromine dichloromethane solution. Hence, an addition reaction takes place an 1,2-dibromo-1,2-diphenylethane is formed.
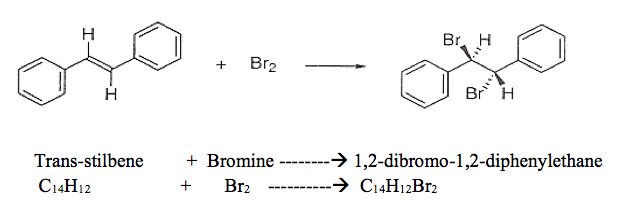
MECHANSIM: The alkene, trans-stilbene, acts as a nucleophile and the bromine acts as an electrophile. Hence, the nucleophilic double bond undergoes an electrophilic addition reaction by the bromine reagent. The Br–Br bond becomes polarized and the more positively charged Br atom is transferred to the alkene to yield a bromonium ion. The addition of bromine begins at one side of the double bond (either side is equally likely, but only one option is drawn) and is followed by attack of bromide ion on the bromonium ion (again, attack could occur at either carbon since the ion is symmetric, but only one option is drawn). The result is a trans dibromide, as shown above.
The product of this reaction, 1,2-dibromo-1,2-diphenylethane, has two stereogenic
centres which normally leads to predict four (2n= 22 in this case) possible
stereoisomers (i.e. four optical isomers). However, there are only three possible isomers in the case of 1,2-dibromo-1,2-diphenylethane since one, the so-called meso isomer, is superimposable on its mirror image.
PROCEDURE:
- 5g of trans-stilbene were added to a 250cm3 conical flask, followed by 40cm3 of dichloromethane. The flask was swirled to dissolve all the stilbene.
- Approximately 15cm3 of 10% bromine-dichloromethane solution were carefully measured.
- 5cm3 of the bromine solution were added to the solution of stilbene and contents were swirled until the reddish brown colour of the bromine solution disappeared.
- A further 5 cm3 of the bromine solution were added to the solution of stilbene and the contents were swirled again until the reddish brown colour of the bromine solution disappeared for a second time.
- The remaining bromine solution was added and the mixture was swirled for several minutes to ensure bromine’s colour persisted.
- Cyclohexene was added drop wise to the flask until the bromine colour disappeared.
- The flask was then cooled in an ice bath and the crystals were collected after carrying out filtration using a Buchner funnel and flask.
- The crystals were washed with 15cm3 cold dichloromethane and were allowed to dry.
- The melting point of the product was determined.
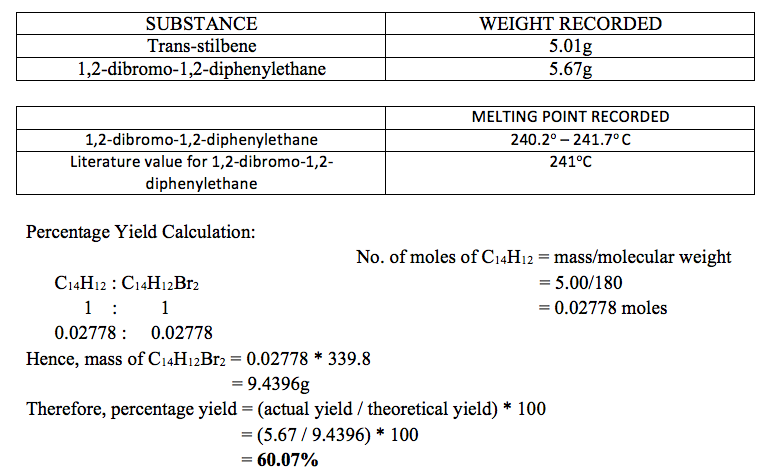
RESULTS AND CALCULATIONS:
Experimental data was recorded in the following tables:
DISCUSSION:
SOURCES OF ERRORS:
- When transferring 5g of trans-stilbene to the conical flask, there was still some trans-stilbene left on the weigh boat. (Systematic Error)
- More crystals would have been formed and a better percentage yield could have been obtained if the flask was left in the ice bath for a bit longer. (Systematic Error)
- All the crystals were not transferred from the Buchner funnel to the petri dish. (Systematic Error)
CONCLUSION:
Trans-stilbene was brominated and 5.67g of 1,2-dibrmono-1,2-diphenylethane were obtained. This is a percentage yield of 60.07%. The melting point range of 1,2-dibromo-1,2-diphenylethane was found to be 240.2o to 241.7o C.
REFERENCES:
- http://fscimage.fishersci.com/msds/58466.htm for literature value of 1,2-dibromo-1,2-diphenylethane (i.e. melting point = 241o C)
- Google images for structural equation of the reaction.
