Reduction of trans-Cinnamaldehyde
By: Jane Huang, Stephanie Thomas and Vasthi Yanes
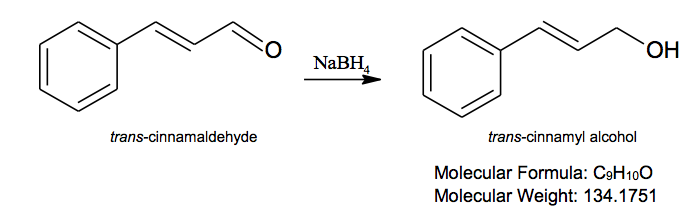
Chemical Reaction Equation
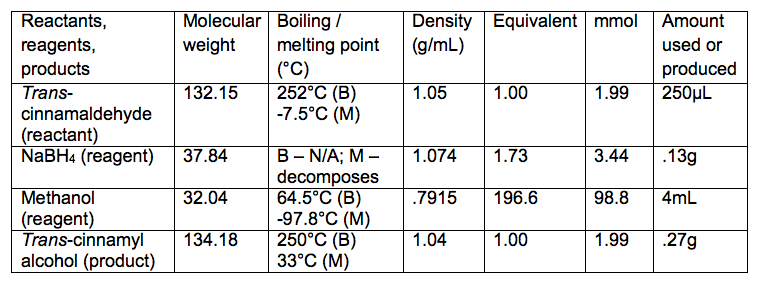
Reaction Table
Introduction
The purpose of this lab is to perform a reduction of trans-cinnamaldehyde. This process consists of 1) mixing trans-cinnamaldehyde with sodium borohydride and methanol, 2) employing rota-vap and the rinsing and removal of aqueous layers to purify mixture, 3) drying the precipitate via gravity filtration.
The use of metal hydrides, such as sodium borohydride and lithium aluminum hydride, is the most commonly used out of three main reduction techniques in labs. In mild acidic conditions, these reagents are capable of reducing most aldehydes and ketones to alcohols. NaBH4 is a selective reagent that reduces aldehydes and ketones only when other functional groups are present. Its reaction mechanism begins with an attack by the hydrogen ion onto the carbonyl carbon. The displaced π bond then attaches to the boron. Since all four hydrogens in the reagent can react, the process continues three more times until the boron is attached to four different carbonyl groups. Methanol then cleaves the tetraalkoxyboron compound to furnish the alcohols.
Safety hazards that should be taken into consideration when performing this procedure include the irritancy of trans-cinnamaldehyde and trans-cinnamyl alcohol, toxicity of methanol, and flammability of sodium borohydride, ethyl acetate, methanol, petroleum ether, and t-butyl methyl ether.
Experimental Sections
- Put magnetic stir bar in 15mL round-bottom flask
- Add 250μL trans-cinnamaldehyde and 4mL methanol
- Secure in ringstand and put in ice-water bath
- Add .13g sodium borohydride using microspatula
- Stir reaction on magnetic stirrer
- Perform reaction in fume hood
- Wait 20 minutes
- Evaporate methanol in rota-vap
- Add 2mL water to residue and 5mL t-butyl methyl ether
- Transfer liquid to srew-cap test tube
- Rinse round-bottom flask with 1mL water and 2mL BME
- Transfer rinse to screw-cap test tube
- Cap tube, shake, and vent
- Remove aqueous layer using Pipet
- Wash organic layer with 2mL saturated solution of NaCl in water
- Dry organic layer with anhydrous magnesium sulfate
- Remove drying agent by gravity filtration
- Collect filtrate in 25mL round-bottom flask
- Evaporate solvent in rota-vap
- Weigh product and calculate yield
Data and Observations
- Color of mixture prior to addition of NaBH4: yellow
- Stirring began at 2:30PM
- Stirring ended at 2:51PM
- White precipitate formed
Theoretical, Actual, and Percent Yields
- Theoretical yield = .27g
- Actual yield = .0957g
- Percent yield = 35.44%
Discussion and Conclusion
The purpose of this lab was to perform a reduction of trans-cinnamaldehyde. The fulfillment of this goal was supported by the appearance of the precipitates formed. Reacting trans-cinnamaldehyde with sodium borohydride and methanol resulted in the formation of a whitish precipitate, which was consistent with the color indicated in the Material Safety and Data Sheet for trans-cinnamyl alcohol. In addition, even though there was an abundance of methanol in the mixture, the lack of a base or acid catalyst hampered the formation of hemiacetals and acetals as a significant alternative organic product. The success of this experiment would be further verified by the results of the 1H-NMR spectra.
NaBH4 was a selective reagent that reduces aldehydes and ketones only when other functional groups are present. Its reaction mechanism began with an attack by the hydrogen ion onto the carbonyl carbon. The displaced π bond then attached to the boron. Since all four hydrogens in the reagent could react, the process continued three more times until the boron was attached to four different carbonyl groups. Methanol then cleaved the tetraalkoxyboron compound to furnish the alcohols.
References
- R. Palleros (2000), Experimental Organic Chemistry, 1st ed., John Wiley & Sons, New York, pg. 445-448, 453-454.
- http://www.sciencelab.com/msds.php?msdsId=9923482
- http://www.sciencelab.com/msds.php?msdsId=9924969
- http://www.sciencelab.com/msds.php?msdsId=9927227
- http://www.sciencelab.com/msds.php?msdsId=9923489
