Transforming Bengay into Aspirin
Transforming Bengay into Aspirin
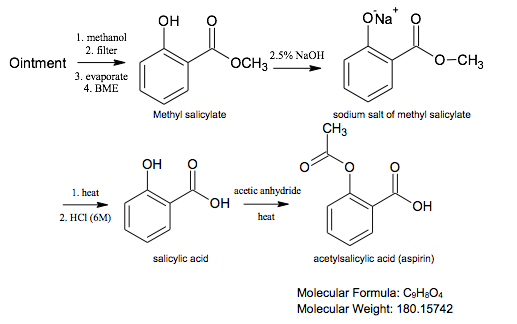
Chemical Reaction Equation
Introduction
The goal of this experiment is to transform bengay into acetylsalicylic acid (aspirin). This process consists of 1) isolating methyl salicylate from the rest of the ointment, 2) hydrolyzing methyl salicylate, and 3) reacting the salicylic acid with acetic anhydride to form acetylsalicylic acid.
Methyl salicylate and menthol are extracted by methanol because the other inactive compounds are not soluble in it. The former is then isolated through acid-base extraction since it readily reacts with bases while the latter does not. The isolated methyl salicylate then undergoes an ester hydrolysis. During saponification, the hydroxide attacks the ester carbon and breaks the π bond. The electrons which were displaced onto the oxygen then attack the same carbon and regenerate the π bond, resulting in the loss of the methoxy group. The newly displaced alkoxide deprotonates the hydroxy group, resulting in an enolate. During acidification, the oxygen anion abstracts a hydrogen proton from hydrochloric acid, forming salicylic acid. This compound is converted to aspirin through acetylation. The hydroxy oxygen attacks the carbonyl carbon and breaks the π bond. The oxygen between the previously attacked carbon and the other carbonyl carbon then abstracts the hydrogen from the hydroxy group. The oxygen anion attacks the carbon it is attached to and regenerates the π bond, resulting in the loss of the carboxylic acid leaving group (—OHCOCH3) and the formation of acetylsalicylic acid.
Safety hazards that should be taken into consideration include the corrosiveness of HCl and NaOH, irritability of menthol, flammability of tert-butyl methyl ether and methanol, and toxicity of HCl, NaOH, and methyl salicylate. Acetic anhydride should also be dispensed in the fume hood as it is a lachrymator.
Experimental Sections
- Weigh 3.5g of ointment in 50mL beaker
- Add 10mL methanol
- Break paste against wall of beaker using spatula for 5 minutes
- Let dry over pre-tared double paper towel in fume hood
- Weigh paste on paper after methanol evaporated
- Transfer liquid in beaker into 25mL round-bottom flask and rota-vap
- Transfer liquid to screw-cap tube (tube 1)
- Rinse round-bottom flask twice with 3mL of BME each time and transfer rinses to tube 1
- Add 2mL of 2.5% aqueous NaOH, cap tube, and invert about 50 times, occasionally venting
- Let layers settle – centrifuge if layers are emulsified and do not separate
- Pipet out lower layer and transfer to tube 2
- Extract BME solution two more times with 2.5mL 2.5% NaOH solution and collect aqueous layers in tube 2
- Wash combined aqueous layer in tube 2 with 2mL BME
- Pipet lower aqueous layer and transfer to 15mL round-bottom flask
- Add 2mL 2.5% aqueous NaOH solution and boiling chip to flask
- Attach microscale water condenser and reflux system directly on a hot plate at medium setting for 25 minutes
- Let solution cool down
- Pipet solution to 50-100mL beaker
- Wash walls of flask with water and transfer to beaker
- Add about 1-2mL 6 M HCl – white precipitate of salicylic acid forms
- Cool beaker in ice-water bath
- Stir liquid with glass rod
- Vacuum-filter suspension using Hirsch funnel for about 5 minutes
- Transfer solid to porous plate and dry by spreading it on the surface and pressing it with a spatula for about five minutes
- Weigh product
- Fill approximately one-half of small crystallizing dish with water and place on hot plate to boil
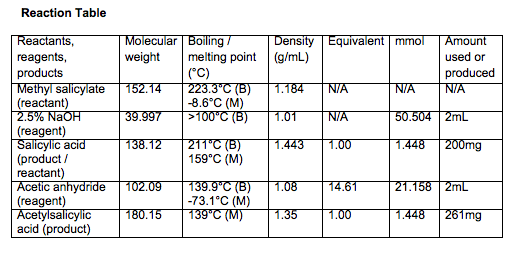
- Weigh approximately 200mg of salicylic acid in 15mL flask
- Add 2mL acetic anhydride
- Add small boiling chip and attach microscale water condenser
- Place round-bottom flask with condenser in the boiling-water bath and let system react for about five minutes
- Add 1mL water through the top of reflux condenser
- Turn off heat and leave for additional five minutes
- Remove water bath from hot place and let system cool down
- Pipet liquid to 50-100mL beaker
- Wash flask with 2mL water and transfer wash to beaker
- Add 4mL water to beaker and cool system in ice-water bath
- Gently scratch walls of beaker with glass rod
- Vacuum-filter aspirin for about 5 minutes
- Transfer to porous plate and dry by spreading it on the surface and pressing with spatula for about 5 minutes
- Weigh product
Data and Observations
- Two layers formed upon addition of BME: one murkier; top layer removed
- After NaOH solution added, gelatinous substance formed—layers not separated
- Some white precipitate formed
- .202g salicylic acid used for aspirin synthesis
- White precipitate formed: .0025g aspirin
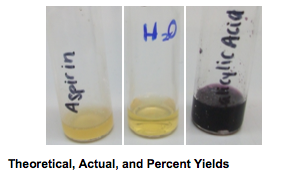
- Ferric chloride test: aspirin and water – yellow; salicylic acid – dark purple
Theoretical, Actual, and Percent Yields
- Theoretical yield = .261g
- Actual yield = .0025g
- Percent yield = .958%
Discussion and Conclusion
The purpose of this lab was to synthesize acetylsalicylic acid (aspirin). The fulfillment of this goal was supported by the ferric chloride test. The dark purple color caused by the salicylic acid sample indicated the presence of phenols in the original mixture while the yellow color caused by the aspirin sample signified that the molecule was no longer a phenol.
The low percent yield of the experiment can be attributed to two factors: 1) the emulsification of the layers after the NaOH solution was added to the mixture and 2) the supersaturated nature of the aspirin solution that inhibited the crystallization of the precipitate. Both issues can be rectified in future experiments by 1) centrifuging the layers in order to induce separation of the emulsified fluids and 2) “seeding” the aspirin solution with pure aspirin in order to induce the formation of the precipitate.
The isolated methyl salicylate proceeded through the following reaction mechanism. Hydroxide attacked the ester carbon and severed the π bond. The electrons which were displaced onto the oxygen then attacked the same carbon and regenerated the π bond, resulting in the loss of the methoxy group. The newly displaced alkoxide deprotonated the hydroxy group, resulting in an enolate. The oxygen anion abstracted a hydrogen proton from hydrochloric acid, forming salicylic acid. This compound was converted to aspirin through acetylation. The hydroxy oxygen attacked the carbonyl carbon and cleaved the π bond. The oxygen between the previously attacked carbon and the other carbonyl carbon then abstracted the hydrogen from the hydroxy group. The oxygen anion attacked its neighboring carbon and regenerated the π bond, resulting in the loss of the carboxylic acid leaving group (—OHCOCH3) and the formation of acetylsalicylic acid.
References
- R. Palleros (2000), Experimental Organic Chemistry, 1st ed., John Wiley & Sons, New York, pg. 473-478, 491-495.
- http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch20/ch20-3-3-1.html
- http://www.sciencelab.com/msds.php?msdsId=9927362
- http://www.sciencelab.com/msds.php?msdsId=9926871
- http://www.sciencelab.com/msds.php?msdsId=9927249
- http://www.sciencelab.com/msds.php?msdsId=9927061
- http://www.sciencelab.com/msds.php?msdsId=9922977
