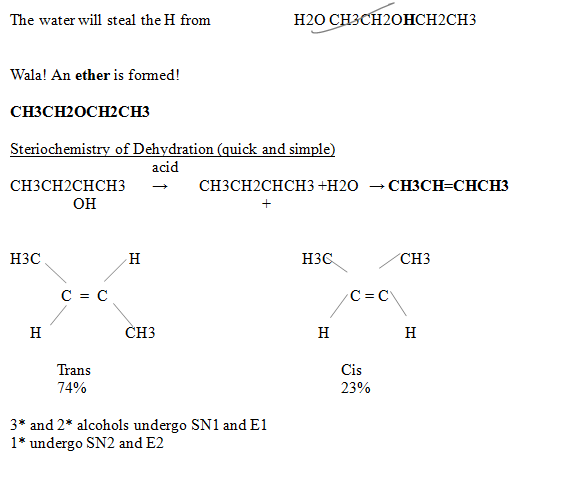
Dehydration of Alcohols
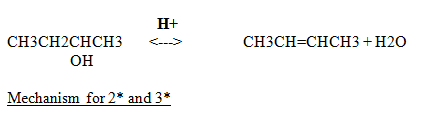
Acid protonates
Converts strong base into weak base (bad to good leaving group)
Water departs forming C+ (rate determining)
Major product is the most substituted (stable) alkene
Tertiary is the easiest to dehydrate because of their C+
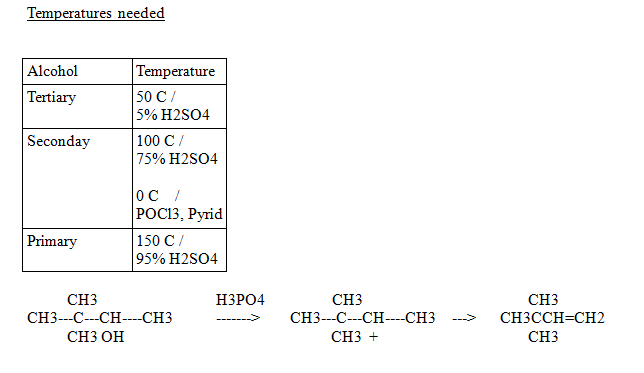
The cation is a secondary cation.
It can rearrange to a tertiary (our best friend) to retain a stable charge
Thus rearranging to a more stable product.
The product above forms abour 2.99% of the time.
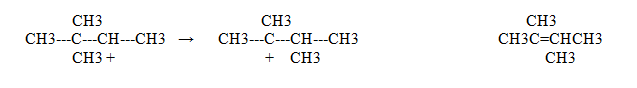
The E2 and SN2 dehydration of primary alcohols can compete.
When the water departs a primary alkane, it can eliminate or substitute for the Alkane
If it eliminates, than we have this product
CH3CH2OH → CH2=CH2
If it substitutes, another alkane-o-hol is added
CH3CH2OH + CH3CH2OH → CH3CH2OHCH2CH3