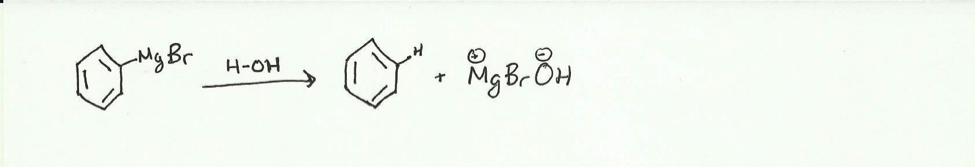Synthesis of Triphenylmethanol from Benzophenone and Bromobenzene
Abstract
The purpose of this lab was to use benzophenone and bromobenzene to synthesize triphenylmethanol. This was done via a Grignard reaction.
The reaction was refluxed to form the Grignard reagent and then recrystallized to obtain a pure product. The melting point of the final pure product was determined to be 160 – 163.2 centigrade and the product showed IR peaks at 3471.83 cm– and 3059.52 cm–. Triphenylmethanol was synthesized with a 9.21 % yield.
Introduction
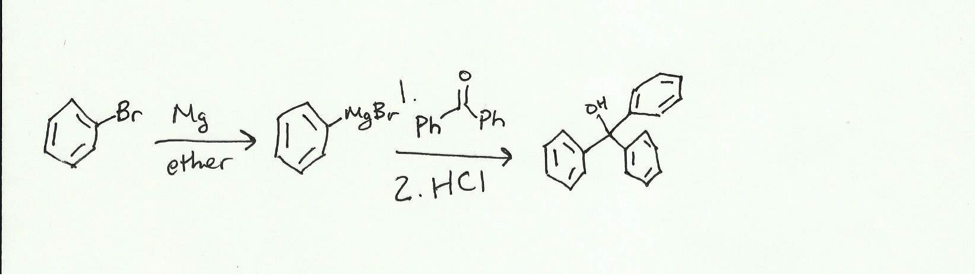
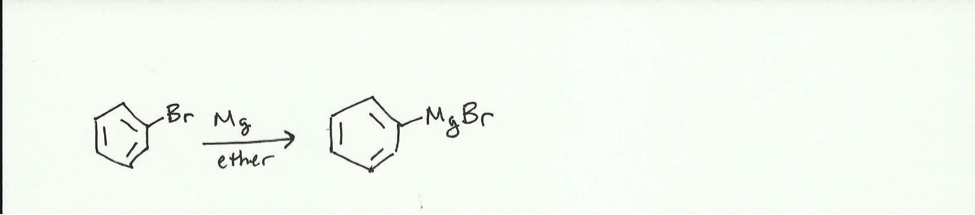
The purpose of the experiment is to synthesize triphenylmethanol from bromobenzene and benzophenone. When magnesium is added to the bromobenzene in ether, a Grignard reagent is formed.
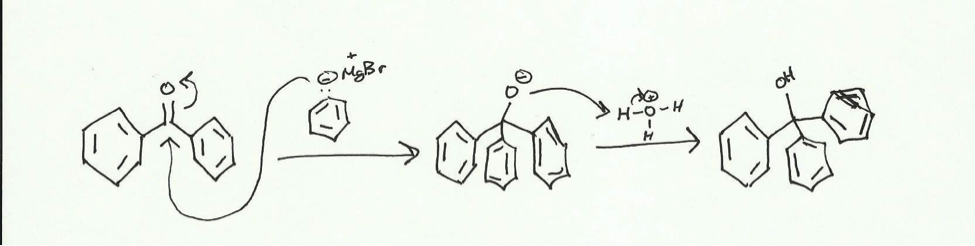
Using this Grignard reagent, triphenylmethanol can be synthesized in a two-step reaction first adding the reagent and then adding an acid.
The Grignard reagent attack the electrophilic carbonyl carbon of the benzophenone and pushed electrons up to the oxygen. The presence of the acid allows for the now negatively charged oxygen to attack a hydrogen to form an alcohol. The reaction will be done in a dry reflux and the product will be obtained via vacuum filtration with a Hirsch funnel. This crude product will be purified using recrystallization in isopropyl alcohol and then collected with vacuum filtration. The product will be characterized using melting point (comparing it to a standard of 162 centigrade) and IR (with standard peaks being broad at around 3600- 3200 cm– and sharp at 3100-3000 cm–.
Procedure
Into a dry 20 mL round bottom flask, .115 g Mg, a dry stir bar, and .8 mL of a 4 mL ether and .777 g bromobenzene solution were added. Reaction mixture was heated above a 60 centigrade hot plate. Remaining 3.2 mL of bromobenzene mixture was added over 15 minute period. Ether was added to reaction mixture to replace what was lost during reflux. After 30 minutes the reaction was removed from heat and cooled to room temperature. 1.09 g benzophenone dissolved in 2 mL of ether was rapidly added to cooled reaction mixture. Reaction mixture stirred until Grignard adduct was fully formed. 6.1 mL of 6M HCl added to adduct to neutralize the mixture. Ether was added to have 10 mL in organic layer of solution. Stirred until all solid had dissolved and lower aqueous layer was removed with a separatory funnel. Organic layer was transferred to Erlenmeyer flask. Aqueous layer was re extracted in the funnel. The organic layer was dried with sodium sulfate and then decanted into a small Erlenmeyer flask. Ether solvent was then evaporated in a hot water bath at 50 centigrade. 3.1 mL petroleum ether (at 50 centigrade) added to oily solution. Product formed collected via vacuum filtration and washed with small portions of petroleum ether. Crude product was then recrystallized with hot isopropyl alcohol and product formed collected via vacuum filtration.
Results and Calculations
Mass crude product = .157 g
Mass pure product = .118 g
Limiting reagent
.777 g benzophenone * (1 mol/ 157.0 g)*(260.3 g/ mol triphenylmethanol) = 1.28 g
Percent yield:
Crude = .157/1.28 *100 % = 12.3 %
Pure = .118/1.28 *100 % = 9.21 %
MP pure product = 160 – 163.2 centigrade
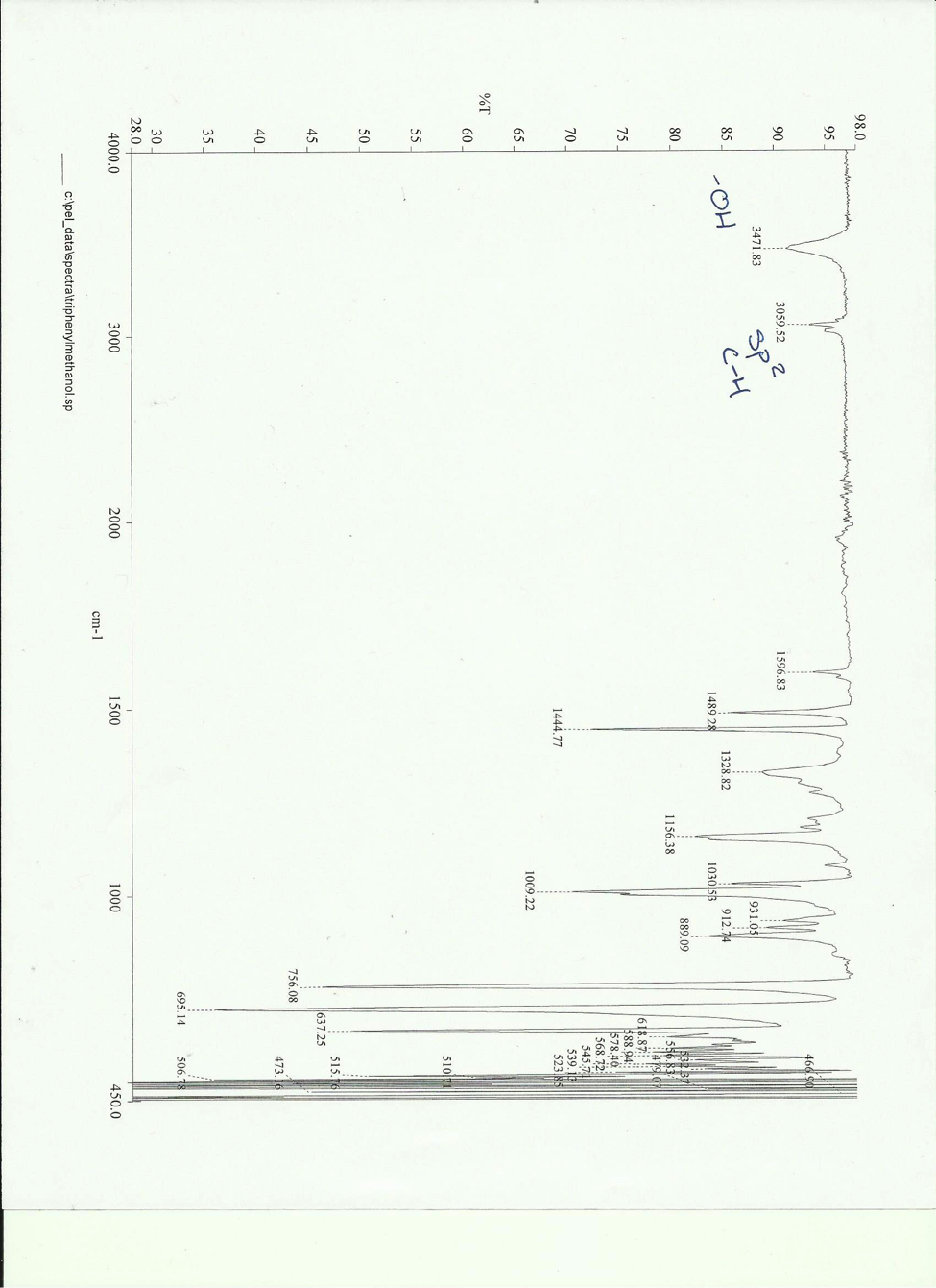
IR peaks of pure = 3471.83 cm– and 3059.52 cm–
Discussion and Conclusion
Using the characterization techniques of IR and melting point it was determined that triphenylmethanol was synthesized. Both of the IR peaks formed in the product IR (3471.83 cm– -OH and 3059.52 cm– sp2 C-H) were characteristic of triphenylmethanol. The melting point of 160 – 163.2 was determined and was expected for the product. Percent yield was low however. This could be due to the Grignard adduct sitting in the lab bench for a week when it could have been reacted immediately after it was formed.
Questions
- Benzene is produced as a side product in Grignard reactions due to the presence of an acidic hydrogen. In this case the acidic hydrogen is most likely water. This acidic hydrogen is much more reactive then the electrophilic carbonyl and will react with the Grignard reagent to produce benzene.
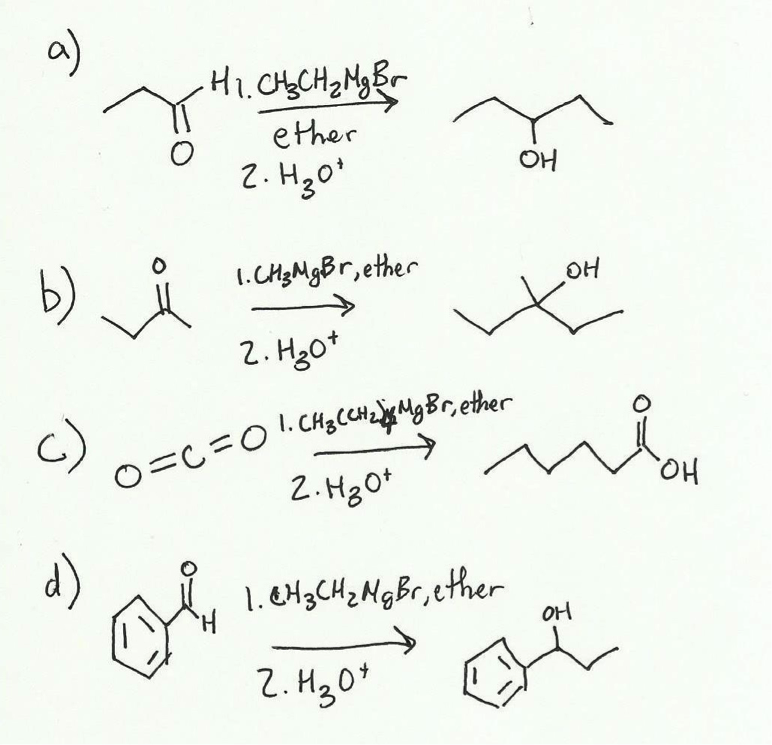
2. Synthesis
Answer these questions:
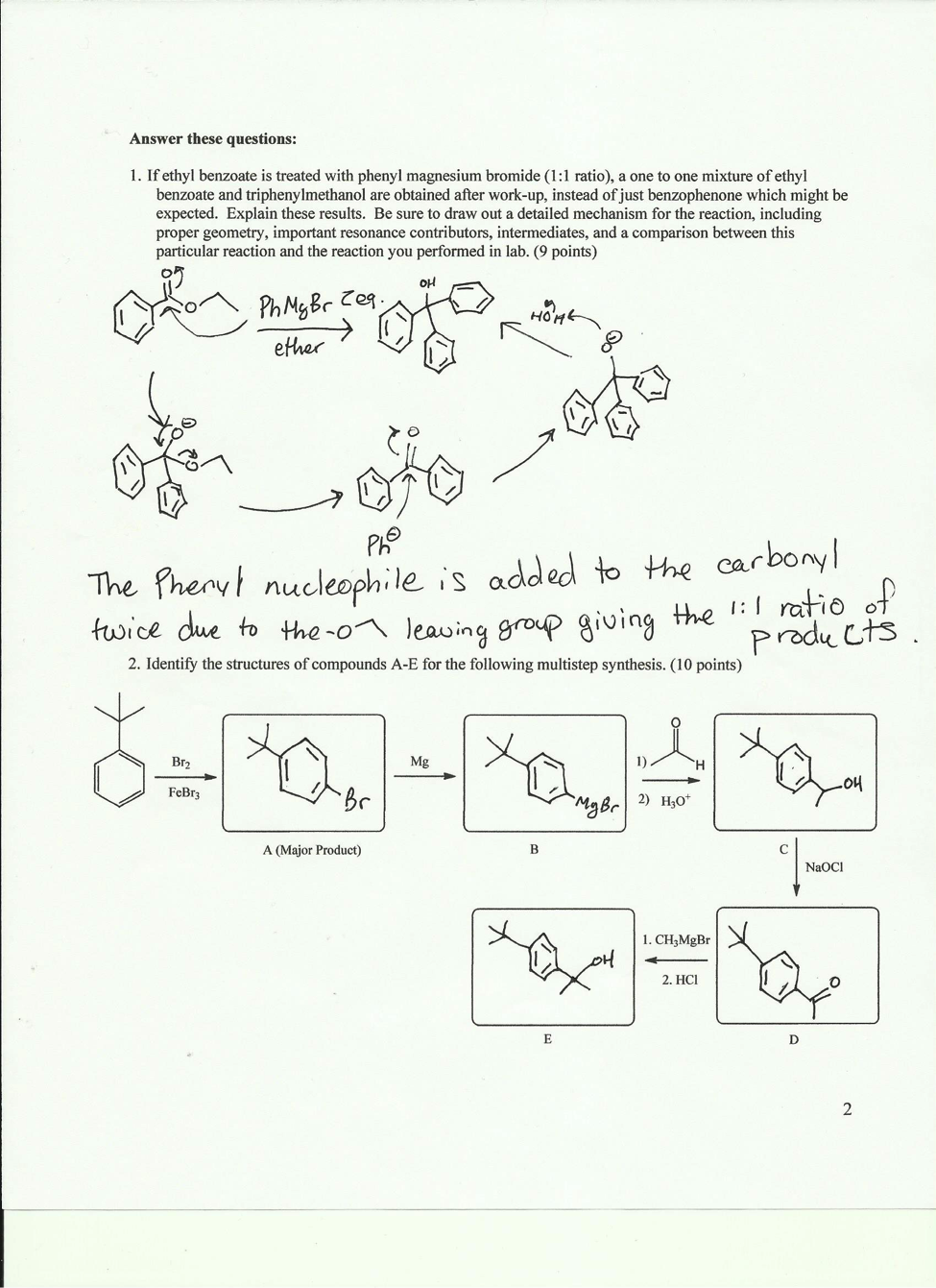
If ethyl benzoate is treated with phenyl magnesium bromide, a one to one mixture of ethyl benzoate and triphenylmethanol are obtained after work-up, instead of just benzophenone, which might be expected. Explain these results.