Recrystallization and Identification of Unknown
Abstract:
In this lab an unknown, contaminated organic compound, “E”, was given to be purified and identified. The compound was identified to be N-Phenysuccinimide, because it had a melting range of 154.3-155.5 degrees Celsius. When the purified compound was mixed with known N-Phenylsuccinimide, the melting range was not depressed at all confirming the results. An appropriate solvent was found among water, acetone, ethanol and toluene by doing small-scale solubility tests. To purify the N-Phenylsuccinimide, it was dissolved in hot ethanol, filtered to remove contaminants, and then cooled to reform crystals that were separated from the ethanol by vacuum filtration. This technique gave a 34.1% yield. The accepted melting range for N-Phenylsuccinimide is 155-157 degrees Celsius, so the separation techniques produced an almost pure sample of N-Phenylsuccinimide.
Introduction:
The goal of this experiment was to identify an impure organic compound. Because each of these compounds has a unique melting range, if the compound could be purified and a melting range determined, the compound “E” could be identified. To be purified the organic compound would have to be separated from the inorganic impurities in the vile. As 1Cooper reports, one way to separate the contaminants from the organic compound is to find a solvent where the organic compound would dissolve at high temperatures, and little to none of the contaminants would dissolve. Once the compound dissolved it can be filtered to remove the impurities. The then pure sample would be placed in a melting range apparatus and heated until the compound turned to a liquid. The range at which this occurs would be used to identify the compound. Because some impurities may dissolve in the solvent, it would be expected that the melting point of “E” be equal or slightly lower than the ranges given.
Results:
Table 1: Melting Range of “E” possibilities.
| Compound | Melting Range © |
| Acetantilide | 112-115 |
| Benzoic Acid | 118-123 |
| Cinnamic Acid | 121-134 |
| Salicylic Acid | 155-160 |
| N-Phenolsuccinimide | 155-157 |
Melting range of possible compounds as reported by 2Nile chemicals.
Table 2: Determination of Appropriate solvent.
| Solvent | Reaction with Compound | Crystals Upon Cooling | ||
| Room Temp | At Boiling Point | |||
| Water | Nothing Dissolved | Nothing Dissolved | N/A | |
| Acetone | Everything Dissolved | Everything Dissolved | No | |
| Toluene | Partial Dissolving | Most Dissolved | Partial | |
| Ethanol | Partial Dissolving | Everything Dissolved | Yes | |
Table 3: Experimental physical numbers
| Mass Compound E | 2.00 g |
| Mass N-Phenolsuccinimide | 0.682 g |
| Percent Recovery | 34.10% |
| Melting Range Recovered N-Phenolsuccinimide | 154.3-155.5 C |
| Melting Range Recovered + Known N-Phenolsuccinimide | 154.3-155.3 C |
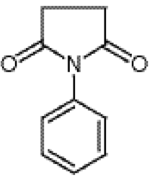
Figure 1: N-Phenylsuccinimide
The figure shows the structure of N-Phenylsuccinimide.
Sample Calculations:
Percent Recovery = 0.682g/2.000g *100
= 34.1 %
Discussion:
The first objective was to determine an appropriate solvent for the impure mixture. This was done using the method described by 3Zubrick. The solvent that worked best for the N-Phenylsuccinimide was ethanol. When added with water, nothing dissolved at all, which is good, but upon heating everything remained solid indicating that water was not an appropriate solvent. The acetone dissolved everything before being heated, which meant that it would not work as a solvent because crystals could not be formed. The toluene partially dissolved the compound at room temperature, and upon heating more dissolved, but there was still a fair amount of what appeared to be the compound that remained at the bottom of the test tube when the heating was done. When it was cooled to recrystallize the compound formed more of a murky solution that it did compact crystals. When ethanol was added originally it appeared as if a small amount of the compound dissolved. When heated up everything but the contaminants remained. Upon cooling there were a lot of crystals that formed and they were all compact at the bottom. This shows that ethanol was the appropriate solvent.
N-Phenylsuccinimide has the configuration of Figure 1, as shown by 2Nile Chemicals. The compound is an organic structure with two ketones around the ring containing four carbons and a nitrogen. The ketone and nitrogen groups would be expected to give the molecule a partial polarity with the negative side going away from the attached benzene ring. Because the general rule in chemistry is “like dissolves like” it makes sense that ethanol is an appropriate solvent. Ethanol is an organic compound that will also be polar because the hydroxide group attached to the ethane backbone is more electronegative than the ethane structure.
The same thing that occurred in the small scale reaction occurred in the large scale test. The two grams of compound “E” was dissolved in 25 mL of hot ethanol. Twenty-Five milliliters was chosen as an amount because it was enough to cover the compound but not much more. When it was filtered hot ethanol was added to the filterpaper and funnel so that crystals would not form as the hot mixture went down the room temperature filterpaper. When the filtration was complete the mixture at the bottom was clear and the filter paper had a lot of contaminants from what appeared to be mini rocks to sawdust to dirt. Crystals formed at the bottom of the flask when it was placed in ice water without any help from seeding or scratching the side of the flask.
The compound was then vacuum filtered to collect the crystals that had formed. The vacuum was set up and the flask was poured directly into the filterpaper. It took about ten minutes before the ethanol stopped dripping through to the bottom. Then the filterpaper was removed and let dry. Upon drying the compound all had the same consistency, it was very fluffy with an offwhite color and the compound formed in little plexiglass looking needles. When the known N-Phenylsuccinimide was collected for the melting point determination, it had the same consistency, which was a good sign that compound “E” was N-Phenylsuccinimide. The mass retrieved was only 0.682g of the original 2.000 g. The contaminants removed from the compound were not weighed, but because of how low the density of the N-Phenylsuccinimide and how much the contaminants weighed, this seemed like a fair amount to recover. Any contaminant that was dissolved in the ethanol would remain dissolved upon cooling, so some mass may have went there. It is possible that mass was lost in transferring the compound from the flask to the other flask when it was filtered.
The compound was then determined by taking melting point as described by 3Zubrick. The compound was placed in a capillary tube inside the melting apparatus and set to melt. At 154.3 degrees Celsius the compounds first drop of liquid formed and by 155.5 degrees Celsius the entire compound was liquid. Comparing these numbers to Table 1 showed that the compound was likely to be N-Phenylsuccinimde, but could possibly have been Salicylic Acid. To verify that the compound was N-Phenylsuccinimide, a 50-50 mixture was prepared of both the purified compound and known N-Phenylsuccinimide. The resulting melting point was determined to be 154.3-155.3 degrees Celsius, close enough to say that the two were the same. If the mixture of the two gave a depressed melting point from the melting point of the known N-Phenylsuccinimide, it would indicate that the compound was salicylic acid instead. The accepted melting point for N-Phenylsuccinimide is 155-157 degrees Celsius. The value observed was lower. This usually indicates that not all impurities were removed during recrystallization, but since the 50-50 mix gave the same melting point, it is more likely the original N-Phenylsuccinimide added to the contaminants was not 100% pure.
Conclusion:
The unknown organic compound “E” was identified to be N-Phenylsuccinimide. Compound “E” was identified by purifying it through recrystallization with hot ethanol as a solvent and determining it’s melting point. It had a melting range of 154.3-155.5 degrees Celsius. When mixed with known N-Phenylsuccinimide, the melting range was determined to be 154.3-155.3 degrees Celsius. The purification techniques performed gave a 34.1% recovery.
Experimental Procedure:
The first procedure was to determine the appropriate solvent for compound “E.” To do this approximately 50 milligrams of the compound was added to four different test tubes. To each of the four test tubes a different solvent was added. Water to the first one, acetone to another, ethanol to another, and finally toluene to the last. The test tubes were then observed for solubility. The ideal solvent wouldn’t dissolve the compound at room temperature, but would upon heating. So each test tube was then placed over a steam bath and brought to a boil. This happened for the water, ethanol, toluene and acetone. The compound was observed and the results recorded. Then the solvents containing the compounds where “E” dissolved upon heating, toluene and ethanol, were placed in an ice bath for ten minutes and observed for crystal formation. The solvent that produced crystals upon cooling was the appropriate solvent, for “E” it was ethanol.
The next goal was to actually purify the compound. To do this 2.000 grams of compound “E” was weighed out and added to a flask. Twenty five milliliters of ethanol at room temperature was added to the flask, and an extra 25 mL was collected in a beaker. Both the flask and the beaker was brought to a boil over the steam bath allowing for the organic compound in the ethanol to dissolve. Once it was dissolved, it was filtered to remove the impurities. Using gravimetric filtration and fluted filter paper that was heated by pouring the extra 25 mL of hot ethanol across it the contents of the flask were filtered. The remaining ethanol and dissolved compound E was placed in an ice bath for ten minutes to allow for the crystals to reform. Then to remove the liquid ethanol from the solid organic compound, vacuum filtration was used. The filter paper containing the solid was allowed to sit in a drawer for a week to dry completely.
One week later the compound was to be identified by taking the melting point and comparing it to that of known compounds. To do this the solid was moved from the filter paper to a clean watchglass that had been zeroed out on a balance to determine the mass of the organic compound collected. Then enough of the compound was added to a capillary tube to produce a 5mm column. The melting range of the compound was then taken by starting the melting apparatus off at 100 degrees Celsius and increasing the temperature at a slow rate (~2 degrees/min) and observing when the compound began to melt and finished melting. Once the melting range was determined, the compound was identified by comparing it to known values (listed above). The compound was thought to be N-Phenylsuccinimide so to verify a 50-50 mix of compound “E” and known N-Phenylsuccinimide was melted in the same procedure listed above.
Resources:
1.“SuperChemLab” http://chemed.eng.clemson.edu/SCL/index.html Clemson University (September 19, 2011)
2. “Nile Chemicals” http://www.nilechemicals.com/ (September 19, 2011)
3. Zubrick, J.W, The Organic Laboratory Survival Manual, John Wiley & Sons, Boston, 2010, 104.