Synthesis and Characterization of Aspirin
Purpose
The purpose of this lab was to synthesize Aspirin and measure the synthesized Aspirin’s purity. By calculating the theoretical yield based on the original amount of Salicylic acid, one could determine the actual yield percentage of the reaction. By measuring the melting point of the synthesized substance as well as taking a UV spectroscopy, it is then possible to measure the purity of the synthesized compound as well. With the yield percentage and the purity of the product, one can get an accurate picture of the efficiency of this process.
Introduction
Organic compounds are a certain type of compounds that contain carbon. The name “organic” is derived from the once-believed assumption that they could only be isolated from plants or made using substances taken from such organic sources.
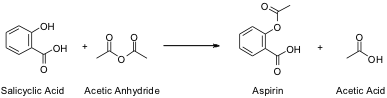
The organic molecule actually synthesized in this experiment is Acetylsalicylic Acid, commonly known as Aspirin. Acetylsalicylic Acid is derived from Salicylic acid, which then reacts with Acetyl Anhydride as per the following reaction in Figure 1.1:
Figure 1.1: Reaction
Reaction of Salicylic Acid and Acetic Anhydride to form Aspirin and Acetic Acid
The rings shown in the Salicylic acid and Aspirin molecules are hexagonal rings of carbon compounds, with alternating single and double bonds as indicated by the double lines. There are Hydrogen atoms bonded to some of the carbon molecules as well, but these are not shown for the sake of simplicity.
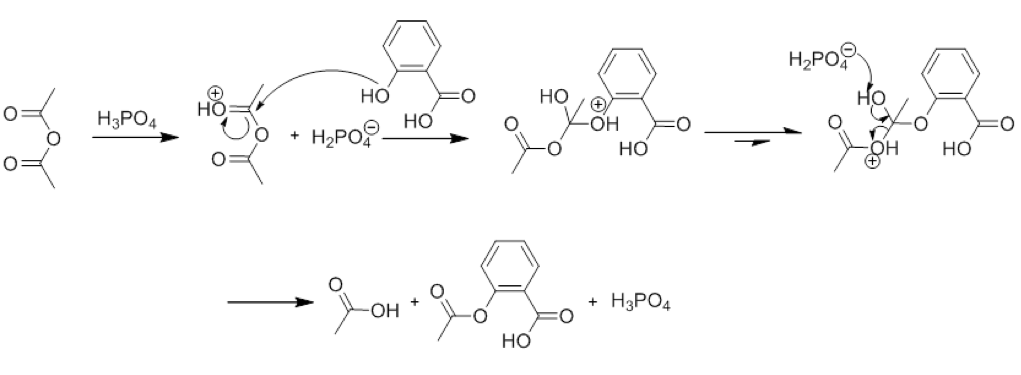
The mechanism of the reaction is more complicated than this, however. Many intermediary steps happen in between the first and last steps, however. These can be seen in Figure 2, which shows the mechanism of the reaction in the presence of H3PO4, a catalyst:
Figure 2: Mechanism of Reaction
Many intermediate steps occur in the reaction
Salicylates have a long history of use to alleviate aches and pain. In 400 B.C Hippocrates documented that extract from the bark of the willow tree (Salix genera) could be used as a remedy for fever, pain, and inflammation, though evidence suggests that people knew about its effects long before. Testing showed that such extract would contain 1.5-12% Salicylic acid and 0.1-20% salicin related derivatives. While these have been shown to be useful to work on symptoms, it had a problem of being too acidic and would irritate peoples’ throats and stomachs. This is partially because of the fact that Salicylic acid is a diprotic acid; this means that when Salicylic acid dissolves in water, it releases two Hydronium ions and makes the solution more acidic than if the same number of a monoprotic acid molecules were added. A monoprotic acid is one that only releases one Hydronium ion when it dissolves in water.
In 1897, Felix Hoffmann discovered that there was indeed a way to make this drug less irritating to the throat and stomach: by reacting Salicylic acid with Acetic Anhydride, it would be possible to acetylate the Salicylic acid to remove one of these proton-donating groups and thus make it less irritating to the body. This resulting compound, Acetylsalicylic Acid, was trademarked by the Bayer as Aspirin®. The “a” in the name “Aspirin” comes from the root acetyl, “spir” from the Greek root “spirea” (willow plant), and “in” is a common medical suffix to help ease of pronunciation. With a name, Bayer proceeded to commercialize the Acetylsalicylic Acid in 1899, though it was not until 1915 that Aspirin was released as an over-the-counter non-steroidal anti-inflammatory drug.
A few concepts are understood to be known in this experiment. The first of these is the concept of a limiting reactant. Whenever two or more reactants are brought together to do something, they react in particular whole-number ratios. As such, any reactant that exists in an amount more than this whole number ratio is known as an excess reactant and some will be left over when the reaction is completely finished. The reactant that is used up in its entirety is known as the limiting reactant. By using molar masses and these whole number molar ratios, it is also possible to calculate the theoretical yield of the reaction. By comparing the actual yield of the reaction and the theoretical yield, one can then find the yield percentage of the experiment. As no experiment is perfect, the yield percent will always be less than 1.
Another chemical concept used in this lab is that of a catalyst. A catalyst is something that changes the rate of a reaction, but is not actually consumed in the reaction itself. In this experiment, the catalyst of Phosphoric Acid is used to catalyze the formation of Aspirin, which can be seen in the mechanism of the reaction in Figure 2. Catalysts do not change the composition of the final product, however, and this is also visible in Figure 2.
Many different laboratory techniques are used in the synthesis of Aspirin. The first of these that is used is the procedure of weighing by difference. To do this, place an arbitrary amount of the substance to be weighed greater than the desired amount on the Fischer balance and press the tare bar to reset the balance to zero. Then, after resetting the balance, take the large arbitrary amount of substance and transfer some of the substance to the desired beaker or other receptacle. By replacing the arbitrary large amount of substance that was on the balance at first, it is then possible to view the total amount of substance removed from the large arbitrary amount and is, consequentially, in the desired receptacle. It is possible to repeat this process in order to end up with the desired amount of substance in the end.
Another laboratory procedure used in this procedure is reading and recording liquid volumes. There are many instruments that can be used to measure liquid amounts that differ in measuring capacity and accuracy, but the one used in this experiment is the graduated cylinder. To measure an amount of a liquid, the liquid to be measured is poured into the graduated cylinder. When liquid is placed in a narrow vertical glass tube, the forces of adhesion and cohesion will cause to form a curve in the tube. This curve, whether the liquid in the middle is higher or lower than the outside, is called the meniscus. In order to get a correct reading while looking at a graduated cylinder, one must look at the middle of the meniscus. If the meniscus curves up, then the correct reading will be at the bottom of the meniscus. Conversely, if the meniscus curves down and forms a sort of dome, the proper measurement is at the top of the meniscus.
Another laboratory technique used in this experiment is vacuum filtration. The process of vacuum filtration uses a filter crucible, an aspirator, and a vacuum hose in order to separate a liquid from a solid. The crucible is placed in the aspirator with a rubber stopper between the two pieces of equipment to ensure the seal between them. Turning the aspirator on will cause a low-pressure area in the vacuum hose, which will in turn pull air and anything else through the crucible that can get through the filter (liquid). Once all of the liquid is gone from the crucible, the solid should be rinsed with a solvent that will not dissolve the solid in order to wash out and trapped impurities. Vacuum filtration can be used to help dry out a sample as well.
Another laboratory technique used in this experiment is recrystallization. This process is necessary because Aspirin is crystalline at room temperature, but when it is synthesized, it is in solution at a higher temperature. Crystallization is how the Aspirin product is isolated from the solution, but it requires the chance formation of a few introductory crystals at the beginning. This initial crystallization can be induced by scratching the side of the beaker: such scratching will dislodge tiny glass particles that can be used as templates to start the crystallization process. Crystallization can also be induced by adding some already-formed crystals from another solution.
A Melt-Temp apparatus is used in this experiment as well in order to find the melting point of the synthesized substance. To use this apparatus, one must use a small glass capillary tube to scoop up a small amount of the substance and then place this capillary tube inside the apparatus. Once this is set up, turning on the apparatus will slowly heat up an aluminum block touching both the capillary tube and a thermometer. By observing the substance and noting at which temperature the substance starts to melt then what the temperature the substance is completely melted, it is then possible to determine the range of the melting point. Different substances have different melting points, as is visible in Figure 3
Figure 3
|
Name |
Formula |
Molecular Weight (g/mol) |
Melting Point (° C) |
Boiling Point (° C) |
Density (g/cm3) |
|
Salicylic Acid |
C7H6O3 |
138.12 |
158.161 |
211 |
1.433 |
|
Acetic Anhydride |
C4H6O3 |
102.09 |
-73.1 |
139.8 |
1.082 |
|
Acetic Acid |
C2H4O2 |
60.05 |
16.5 |
118.1 |
1.049 |
|
Phosphoric Acid |
H3PO4 |
98.00 |
42.35 |
158 |
1.885 |
|
Acetylsalicylic Acid |
C9H8O4 |
180.157 |
135 |
140 |
1.40 |
Table showing information about the molecules used in the experiment.
A UV-Vis Absorbance Spectroscopy is also used in this experiment in order to determine the absorbance of the sample and, in turn, determine purity. This is done by weighing out a carefully measured amount of product and fully dissolving it in a certain amount of water. If some of this resulting solution is placed in a cuvette and is analyzed by the UV-Vis machine, it is then possible to determine the concentration of the dissolved substance from the given absorbance values using Beer’s law.
In this experiment, a very (but not perfectly) pure sample of Acetylsalicylic Acid should be formed. While some of the formed product will be lost to transferring of product, purification of product, and human error, it still should be reasonable to expect a 50% of the theoretical yield to result from the experiment. While many variables can change in the experiment, there are many variables that are independent (controllable) as well. These independent variables are the amounts of Salicylic acid, Acetic Anhydride, water and any other substances used in the experiment, the temperature of the water used in the experiment, the time spent performing certain tasks, and any other variable that is controlled by the user. These are separate from dependent variables, whose name is derived from the fact that these variables depend on the independent variables. The dependent variables in the experiment are the variables that are not completely controllable: percent yield, speed of crystallization, co-crystallization of Salicylic acid or Acetic Acid, or anything else not changeable by the person running the experiment. This subset of variables is completely different from the things that remain constant in the experiment: regardless of anything else that happens, water will always have the same absorbance, all chemicals used will have the same molecular mass, density, melting point, and other physical quantities, and the equipment used in the experiment will always act the same way. All of the physical characteristics of the different chemicals in Figure 3 are constants, as well. Changing a variable will not affect these constants.
Procedure
In this reaction, an excess of Acetic Anhydride (C4H6O3) is reacted with a carefully measured amount of Salicylic acid (C7H6O3) in the presence of Phosphoric Acid (H3PO4), a catalyst. The mixture is heated to form the Acetylsalicylic Acid (C9H8O4) and Acetic Acid (C2H4O2). After the reaction takes place, water is water is used to cleanse the excess Acetic Anhydride from the product and cause the Aspirin product to crystallize. After collecting this Aspirin product, it is then purified by recrystallization, and its melting temperature measured in order to determine its purity. A small, carefully controlled amount of the product is dissolved in water and this dilute solution is then analyzed with UV-Spectroscopy.
Materials and Equipment
-Acetic Anhydride
-aspirator
-aspirator
-beakers (100ml, 200ml, 400ml)
-Capillary tubes
-cuvette
-Desi-cooler
-distilled water
-eye dropper
-filter crucible
-filter paper
-fume hood
-glass stirring rod
-glass stopper
-glass vial
-graduated cylinders (10mL and 50 mL)
-hot plate
-ice
-lab notebook
-label for watch glass
-Melt-Temp apparatus
-mortar and pestle
-oven
-Phosphoric Acid
-salicylic acid
-scoopula
-squirt bottle
-top-loading balane
-UV-Vis Absorbance Spectrometer
-vacuum hose
-watch glass
-weighing bottle
Procedure
Obtain a filter crucible and a mortar and pestle for later on in the experiment. Using the upright balances, weigh out 2.99 g of Salicylic acid into a 100 mL beaker. Take this Salicylic acid to a fume hood, along with both graduated cylinders, the wash bottle filled with distilled water, the medicine dropper, and a 150 mL beaker to hold distilled water. While working inside the fume hood, add 9 mL of Acetic Anhydride to the Salicylic acid, followed by 15 drops of 85% Phosphoric Acid solution. After doing this, heat the beaker gently until it begins to boil, at which point remove it and allow it to sit for three minutes. After waiting for the beaker to cool, slowly add 40 to 50 drops of distilled water to the mix. When the solution cools, add 30 mL of distilled water to the beaker and swirl the beaker to mix the contents well. If the mixture becomes cloudy or crystals start to appear, reheat the contents again and allow the beaker to cool down again. Once the beaker is cool enough to handle, take it back to the lab bench and attempt to induce crystallization by scratching the side of the beaker with the stirring rod. After scratching the beaker, leave the beaker in an ice bath for 15 minutes. Swirl the beaker occasionally during this 15 minutes to help promote crystallization. While the Aspirin is crystallizing, chill 60 mL of distilled water in a second ice bath.
After the 15 minutes are done, use the filter crucible, water aspirator, and plastic filter flask to isolate the Aspirin crystals from the liquid. Remove excess water after filtering by pressing the water out with a stopper atop a piece of filter paper. Wash the resulting Aspirin with water several times in order to remove excess Acetic Acid. Clean the reaction beaker to remove Acetic Acid from it, and then return the Aspirin crystals from the filter crucible to beaker. Rinse the filter crucible and place it back on the aspirator. Rinse the Aspirin in the filter crucible by putting 10 mL of chilled water in the aspirator, stirring it well, and then allowing the water to be quickly suctioned away. Repeat this rinsing three times, or until the smell of Acetic Acid is no longer present in the solid
After the final washing and rinsing, dry the crystals well. Leave the filtered crystals undisturbed in the filter crucible for 10 minutes with the water aspirator on. After this, lightly stir the crystals for another 5 minutes, again with the water aspirator on. After this vacuuming, spread out the Aspirin evenly on a labeled watch glass and put it in the oven for 30 minutes. While waiting for the Aspirin to dry label a vial and weigh it using a top-loading balance. When the Aspirin is done drying, transfer it to the mortar and use the pestle to grind it into a fine powder. Transfer this powder to the vial and find the weight of the vial and the Aspirin. Store the Aspirin in the Desi-cooler and determine the percent yield of the synthesis by taking the actual yield and dividing it by the theoretical yield, determined from the original amount of Salicylic acid used.
To verify the identity of the Aspirin, completely dissolve .0204 g of Aspirin product in 100 mL of distilled water. Fill a cuvette with distilled water to act as a calibration constant, and then rinse another cuvette with the solution 3 times before filling it with the Aspirin solution. Take both cuvettes to the UV-Vis spectrometer and record the absorbance values at 273 nm and 298 nm. If either of these values is above 1, dilute the solution in a 1:1 ratio in order to keep the absorbance below 1. Record these values and, using Beer’s law, determine the concentration of Salicylic acid and Acetylsalicylic Acid in the solution.
Data and Observations
Figure 4
| Object | Mass (g) |
| Initial Mass of Salicylic Acid | 2.99 |
| Mass of Empty Vial | 23.11 |
| Mass of Vial and Aspirin | 25.81 |
Masses of materials and equipment in synthesis of Aspirin
Figure 5
| Mass of Dissolved Aspirin | .0204 g |
| Volume of Water Added | 100 mL |
| Dilution Factor | 1 : 1 |
| Beginning of Melting Point | 129° C |
| End of Melting Point | 134° C |
Quantitative observations made in the characterization of Aspirin
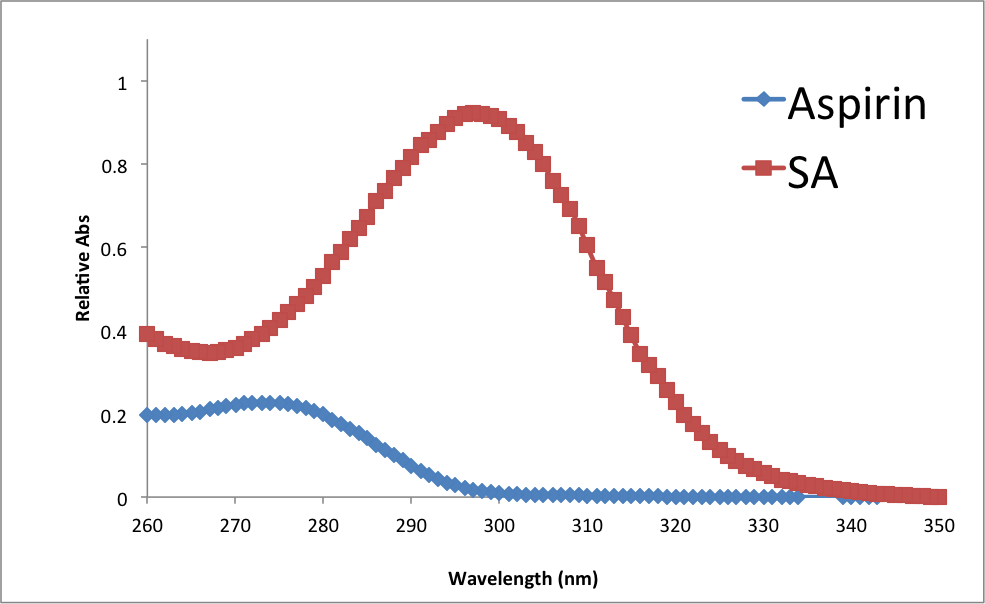
Figure 7
Absorbance spectra of Aspirin and Salicylic Acid
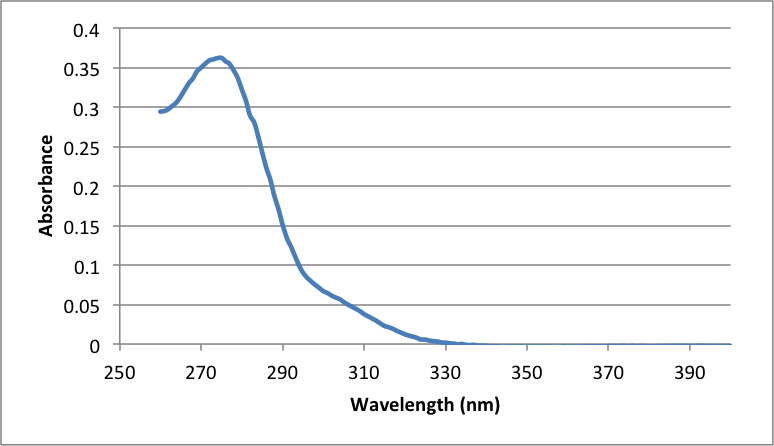
Figure 6
Graph showing the absorbance of the 1:1 dilution of the Aspirin solution used in the UV-Vis Spectroscopy, with A273 = .3602 and A298 = .0737
Calculations
Mass of Aspirin:
MAspirin = Mvial + Aspirin – Mvial = 25.81 g – 23.11 g = 2.60 g
Theoretical yield of Aspirin:
Theoretical Yield of Aspirin = (2.99 g Salicylic Acid) (1 mol Salicylic Acid / 138.12 g Salicylic Acid) (1 mol Aspirin / 1 mol Salicylic Acid) (180.157 g Aspirin / 1 mol Aspirin) = 3.90 g Aspirin
Percent Yield of Aspirin:
Percent yield = (actual yield / theoretical yield) * 100 = (2.60 g / 3.90 g) (100) = 66.7% yield
Purity of Aspirin using Beer’s law:
A273 = (εSA273 x CSA273) + (εASA273 x CASA273)
A298 = (εSA298 x CSA298) + (εASA298 x CASA298)
εSA298 = 3321 M cm-1 εASA298 = 5.701 M cm-1
εSA273 = 867.6 M cm-1 εASA273 = 720.3 M cm-1
-molar absorptivity of Aspirin at 298 nm in near 0 so that factor can be eliminated
A298 = (εSA298 x CSA298)
(A298) / (εSA298) = CSA298 = CSA273 = (C mol Salicylic acid) / (1000 mL)
Mass Salicylic acid = (C mol Salicylic acid) / (1000 mL) * 138.123 g mol-1 Salicylic acid * (100 mL + 100 * dilution factor)
= (.0737) / (3321 g mol-1) * 138.123 g mol-1 Salicylic acid * (200 mL) / 1000 = .0006131 g Salicylic acid
MassAspirin = masssample – masssalicylic acid = .0204 g – .00061305 g = .0198 g Aspirin
Purity of Aspirin = massAspirin / massproduct = .0198 g Aspirin / .0204 g product = 97.0 % Aspirin
Analysis
There are many observable trends in this experiment. Looking at the UV –Vis results, it is possible to assume that larger molecules have higher absorptivity at smaller wavelengths. Looking at results from the Melt-Temp apparatus, it appears that more impure samples have longer, less precise melting points. Regarding the process of recrystallization, it appears that agitation, shock, and low temperature can all help the recrystallization proceed at a faster rate.
Conclusion
The hypothesis of making a reasonably pure sample at a relatively high efficiency stayed mostly true: though not medicinal quality, a purity of 97.0% is not too bad. The efficiency (66.7%) could be improved upon, however. There are a few sources of error in this particular experiment. One notable source of error is the amount of product wasted in transfers from one piece of equipment to another. Another possible source of error is the squeezing process: it is possible to rip the filter paper by pressing too hard with the stopper and thus need to discard some product. Spilling of product or solution is another source of error, along with other mistakes due to human error, as are dust or any other environmental conditions that could impair the results of the experiment. There are a few possible sources of error in the experiment as it stands.
Though the experiment proved the hypothesis to stay true, several improvements could have been made to the procedure. Firstly, the amount of materials could be increased. Because of the relatively small amount of product, small losses in product caused by the many transfers of material are greatly magnified in the percent yield. This could be rectified by using a greater amount of Salicylic acid and Acetic Anhydride in the beginning of the experiment. This experiment could also be made better by reducing the number of transfers between containers: for example, the experiment calls for transferring the product from the filter crucible to the beaker, rinsing the filter crucible, then transferring the product from the beaker back to the filter crucible. It may be possible to simply leave the product in the crucible and rinse it directly after squeezing the excess liquid out, thus reducing the amount of lost product due to transfers. One can reduce the number of transfers by forgoing the step involving the mortar and pestle: though the product will not be a fine and powdery as it could otherwise be, much of the rinsed product is wasted in both the transferred and the act of pulverizing the product as well. Another area in which the experiment could be improved upon is the drying: rather than simply letting the product sit in the crucible to dry, it is possible to stir it constantly in order to help release any trapped liquid escape. The experiment could be improved upon in many ways.
Summary
Much was learned in this experiment. One of the more obvious things seen is the inefficiency of small-scale synthesis reactions. Very similar amounts of work are put in for a very small (as in this experiment) amount of product and a more reasonable (five or six times this experiment) amount of product and less product is lost as well. With specialized tools, it is probable that efficiencies of 90% or more are possible in large-scale, commercial applications of the synthesis of Aspirin. Another important thing that can be gleaned from this experiment is the use of a catalyst to speed up a reaction. While all reactions will eventually take place, it is usually economically feasible if not practical to use an amount of a catalyst to speed up a reaction to an appreciable rate. This can be observed in both the use of Phosphoric Acid in the synthesis part of the experiment and the use of scratching the side of the beaker to induce crystallization.
This experiment also taught the importance of diligence and following directions carefully. If one were to have simply added 40 mL of water to the beaker immediately after boiling it, then the Salicylic acid would have likely co-crystallized with the Aspirin and this would have had very negative results for the rest of the characterization. Given a more dangerous setting, misreading or disregarding directions could result in injury, or worse.